Struggling with your covalent bonding worksheet? You’re not alone! Covalent bonding, the sharing of electrons between atoms, can be a tricky concept to grasp. Many students find themselves bogged down in Lewis structures, understanding electronegativity differences, and predicting molecular shapes. That’s why having a clear understanding of the answers, along with the underlying principles, is crucial for success in chemistry. This post provides a glimpse at common covalent bonding worksheet answers and offers insights to help you better understand the concepts behind them. Remember, simply memorizing answers isn’t the goal; understanding why those answers are correct is the key to mastering covalent bonding and building a solid foundation in chemistry.
This guide isn’t just about providing a quick fix for your homework. We aim to clarify the reasoning behind each answer, offering explanations and examples that will boost your confidence and improve your problem-solving skills in future chemistry assignments. We’ll cover common questions about identifying covalent compounds, drawing Lewis structures, predicting molecular geometries using VSEPR theory, and determining polarity of bonds and molecules. By understanding these fundamental aspects, you’ll be well-equipped to tackle any covalent bonding challenge.
Common Covalent Bonding Worksheet Topics
Before diving into specific answers, it’s important to recognize the typical topics covered in covalent bonding worksheets. These often include:
- Identifying compounds that form covalent bonds (typically between nonmetals).
- Drawing Lewis structures to show the arrangement of atoms and bonding electrons.
- Determining the number of bonding and non-bonding (lone pair) electrons.
- Predicting molecular geometry using VSEPR (Valence Shell Electron Pair Repulsion) theory (linear, bent, trigonal planar, tetrahedral, trigonal pyramidal, etc.).
- Understanding electronegativity and predicting bond polarity (polar vs. nonpolar covalent bonds).
- Determining the overall polarity of a molecule based on its shape and bond polarities.
Knowing these topics will help you anticipate the types of questions you’ll encounter and focus your study efforts. Each area builds upon the previous one, so a strong understanding of Lewis structures, for example, is essential for applying VSEPR theory correctly.
Sample Covalent Bonding Worksheet Answers (Examples)
Here are some example questions and answers, presented in an HTML list format for clarity and ease of access. Remember to use these as a guide, not just as copy-and-paste solutions. Try to understand the reasoning behind each answer.
- Question 1: Draw the Lewis structure for methane (CH4).
Answer:
- Carbon is the central atom with four valence electrons.
- Each hydrogen atom has one valence electron.
- Carbon shares one electron with each of the four hydrogen atoms, forming four single covalent bonds.
- The Lewis structure shows carbon in the center, with four single lines (representing single bonds) extending to each hydrogen atom.
- Question 2: What is the molecular geometry of water (H2O)?
Answer:
- The central oxygen atom has two bonding pairs and two lone pairs of electrons.
- According to VSEPR theory, this arrangement leads to a tetrahedral electron geometry.
- However, the *molecular* geometry, considering only the atoms, is bent or angular.
- Question 3: Is carbon dioxide (CO2) a polar or nonpolar molecule? Explain.
Answer:
- The electronegativity difference between carbon and oxygen makes the C=O bonds polar, with oxygen being more electronegative.
- The molecule is linear.
- The two bond dipoles are equal in magnitude and opposite in direction, canceling each other out.
- Therefore, CO2 is a nonpolar molecule.
- Question 4: Identify which of the following compounds contains covalent bonds: NaCl, H2O, MgCl2, CH4.
Answer:
- NaCl and MgCl2 are ionic compounds (metal and nonmetal).
- H2O (water) and CH4 (methane) are covalent compounds (formed between nonmetals).
- Therefore, the answer is H2O and CH4.
- Question 5: Draw the Lewis structure for carbon tetrachloride (CCl4) and state its molecular geometry.
Answer:
- Carbon is the central atom.
- Each chlorine atom has 7 valence electrons.
- Carbon forms four single bonds with four chlorine atoms. Each chlorine has three lone pairs of electrons.
- The molecular geometry, according to VSEPR theory, is tetrahedral.
These examples illustrate the types of questions you’re likely to encounter and the level of detail expected in your answers. Practice drawing Lewis structures and applying VSEPR theory until you feel comfortable with these concepts.
Remember that understanding the underlying principles of covalent bonding is far more important than simply memorizing answers. By actively engaging with the material, practicing problem-solving, and seeking clarification when needed, you’ll gain a deeper understanding of chemistry and achieve greater success in your studies. Good luck!
If you are looking for WS – Drawing Covalent Bond Diagrams PDF – Name: Class: Date you’ve came to the right place. We have 20 Pics about WS – Drawing Covalent Bond Diagrams PDF – Name: Class: Date like Covalent Bonding Worksheet Answers – Pro Worksheet, Covalent Bonding Worksheet Answers – E-streetlight.com and also Covalent Bonding Worksheet – Wendelina. Read more:
WS – Drawing Covalent Bond Diagrams PDF – Name: Class: Date

worksheets.clipart-library.com
Bonding Basics Worksheet Answers – Printable Word Searches

davida.davivienda.com
Covalent Bonding Naming Worksheet

manualpohovanuosu.z21.web.core.windows.net
Chemical Bonding-answers – Chemical Bonding Worksheet Ionic Bond
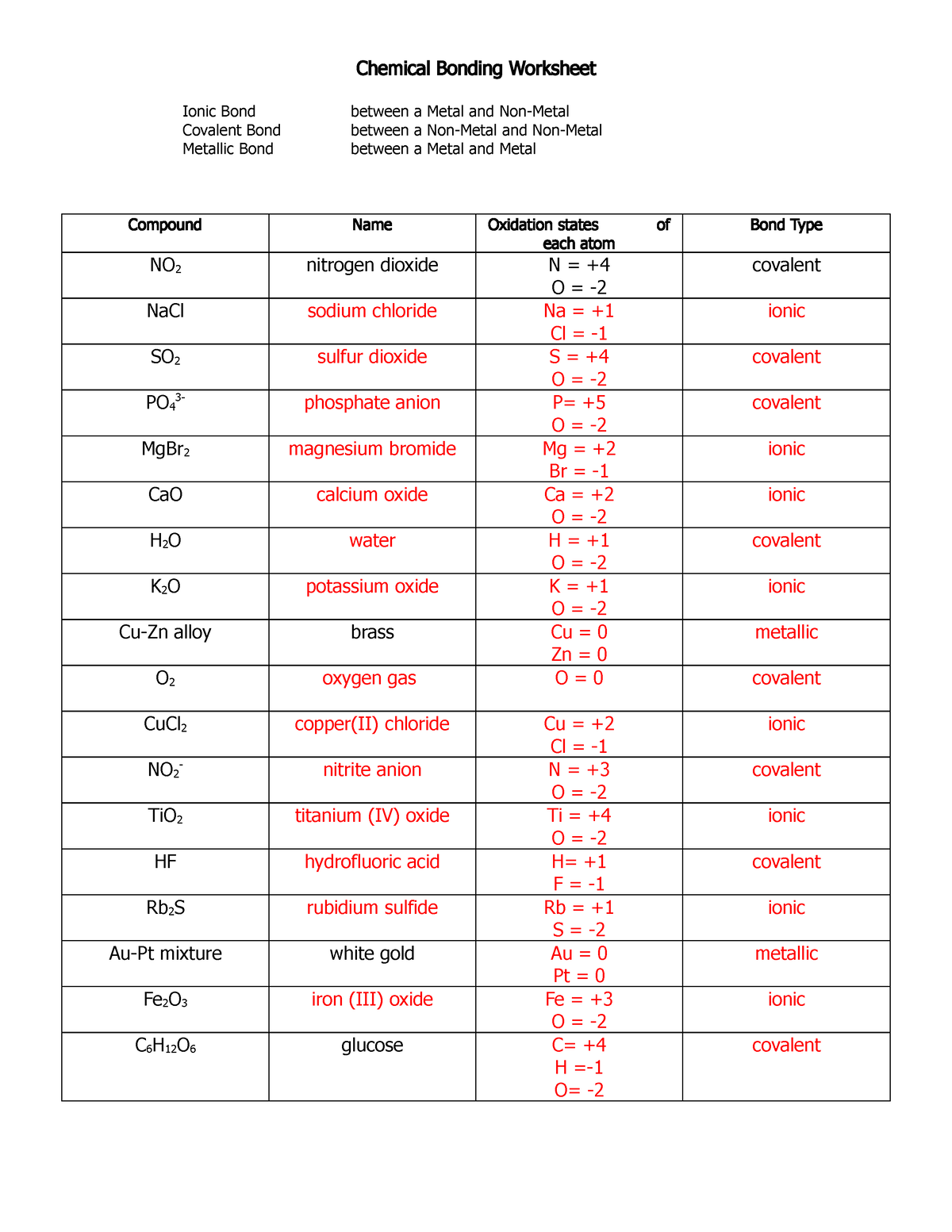
worksheets.clipart-library.com
Homework WS3 Covalent Bonding Answerkey – Name: Date: – Studocu

worksheets.clipart-library.com
Ionic And Covalent Bonds Worksheet With Answers – Worksheets For

worksheets.ekocraft-appleleaf.com
Covalent Bonding Worksheet Answers – Pro Worksheet
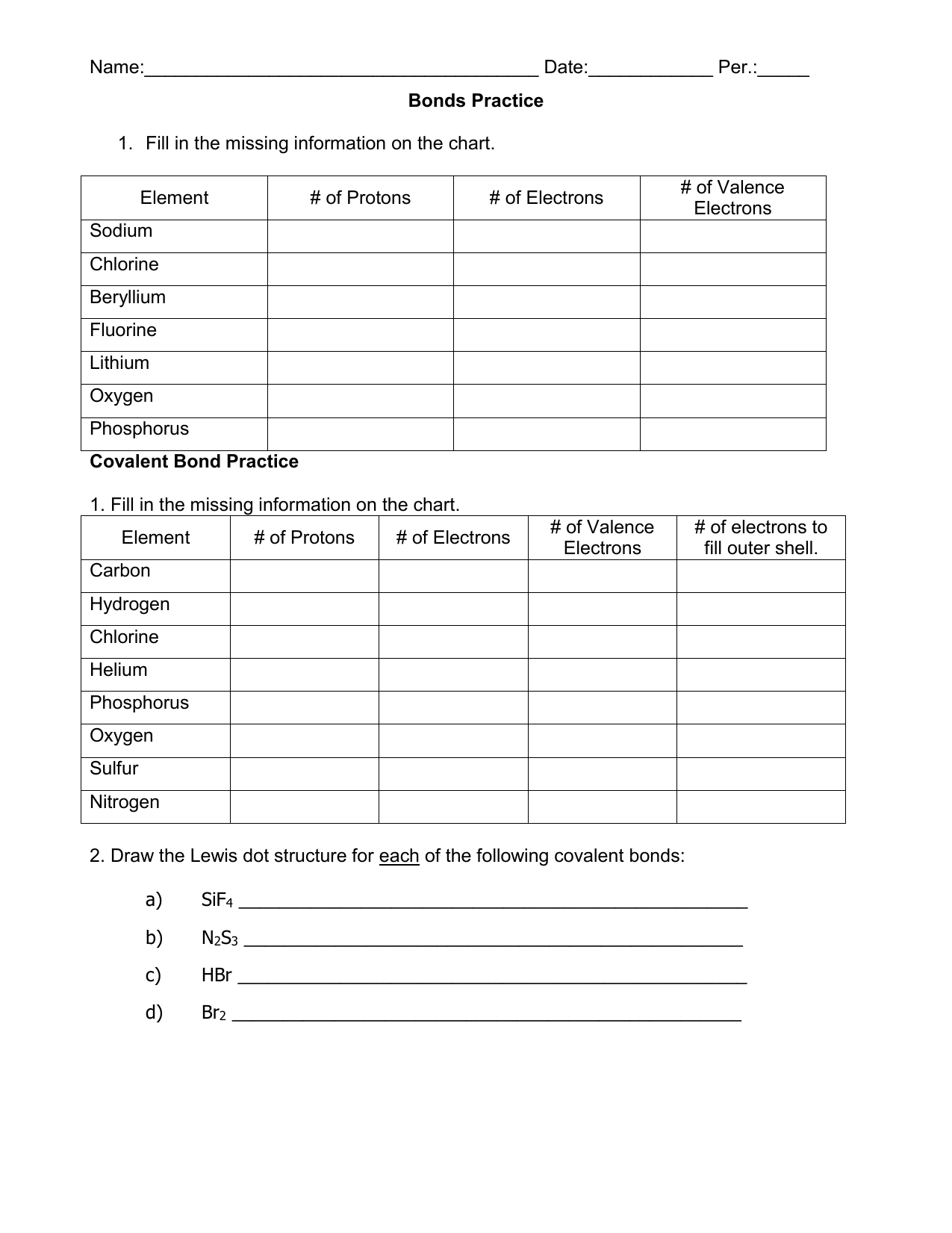
www.proworksheet.my.id
Covalent Bonding Naming Worksheet

manualpohovanuosu.z21.web.core.windows.net
Covalent Bonding Worksheet Answers – E-streetlight.com

www.e-streetlight.com
Simple Covalent Bonding Worksheets Lewis Electron Dot Structures

worksheets.clipart-library.com
Ionic And Covalent Compound Worksheet – CompoundWorksheets.com
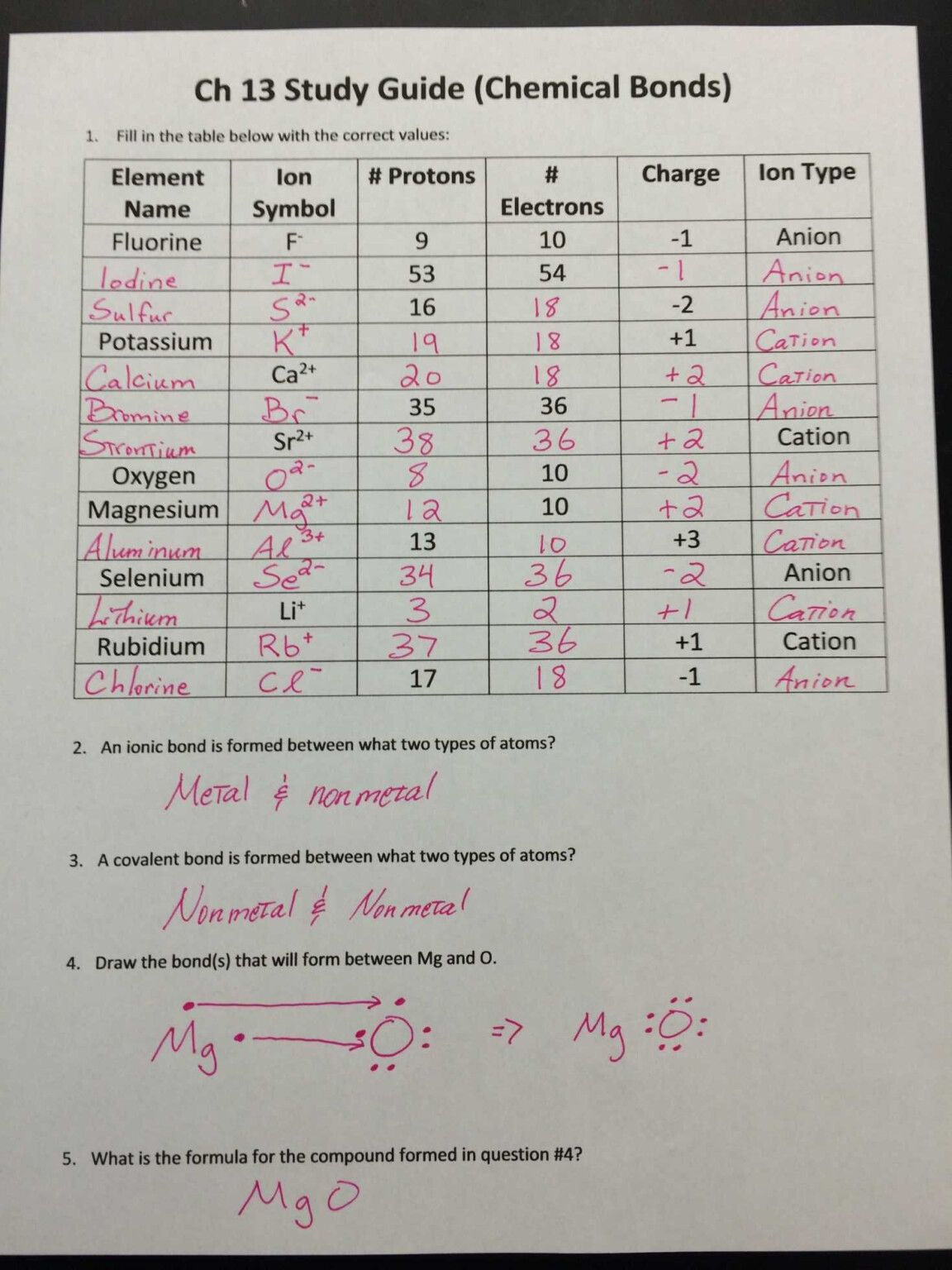
www.compoundworksheets.com
Chemical Bonding Worksheet With Answers Pdf
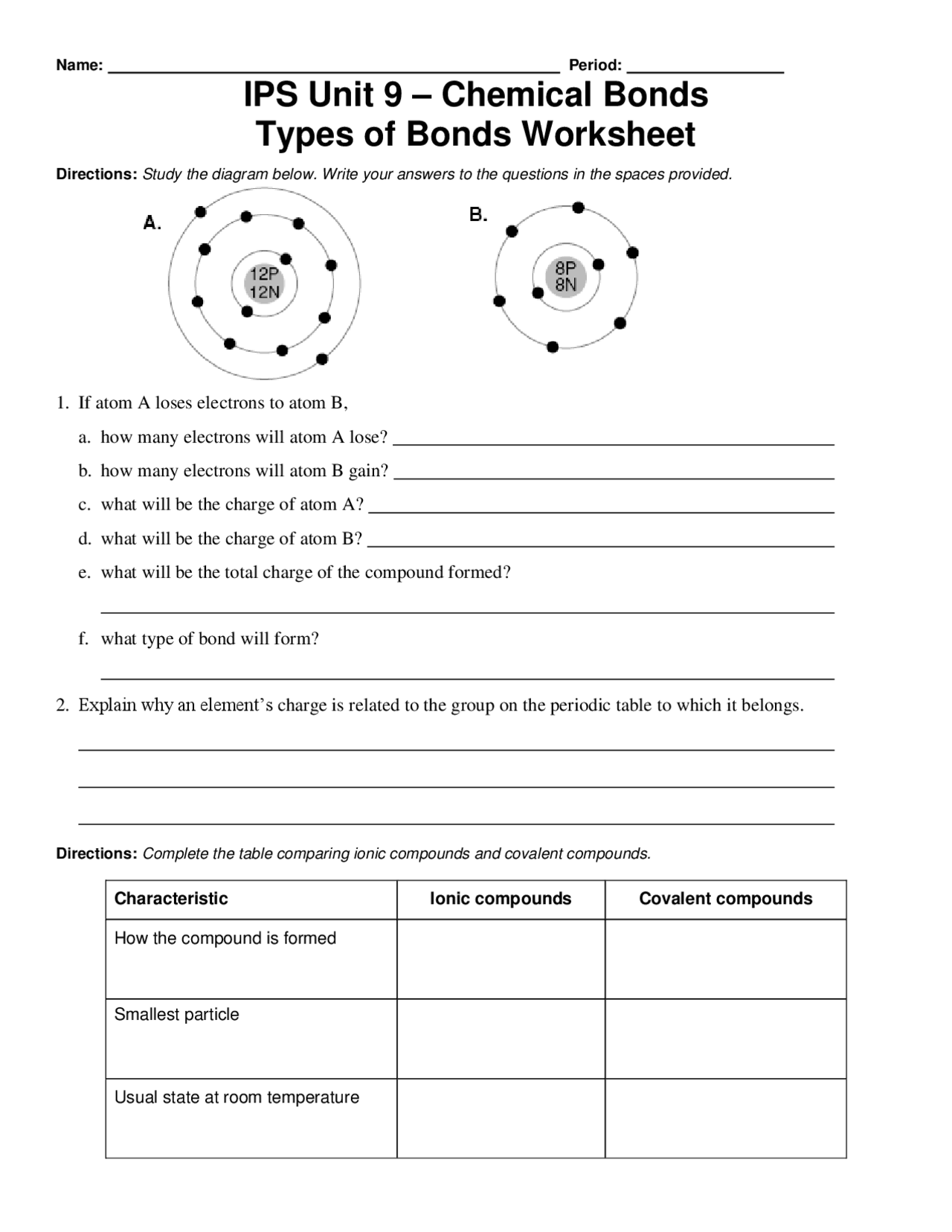
hrpicakt7school.z21.web.core.windows.net
Bonding Worksheet Answers Key List The Following Ionic Compounds

www.compoundworksheets.com
Covalent Chemical Bonding Worksheet Answers – Inspirevio
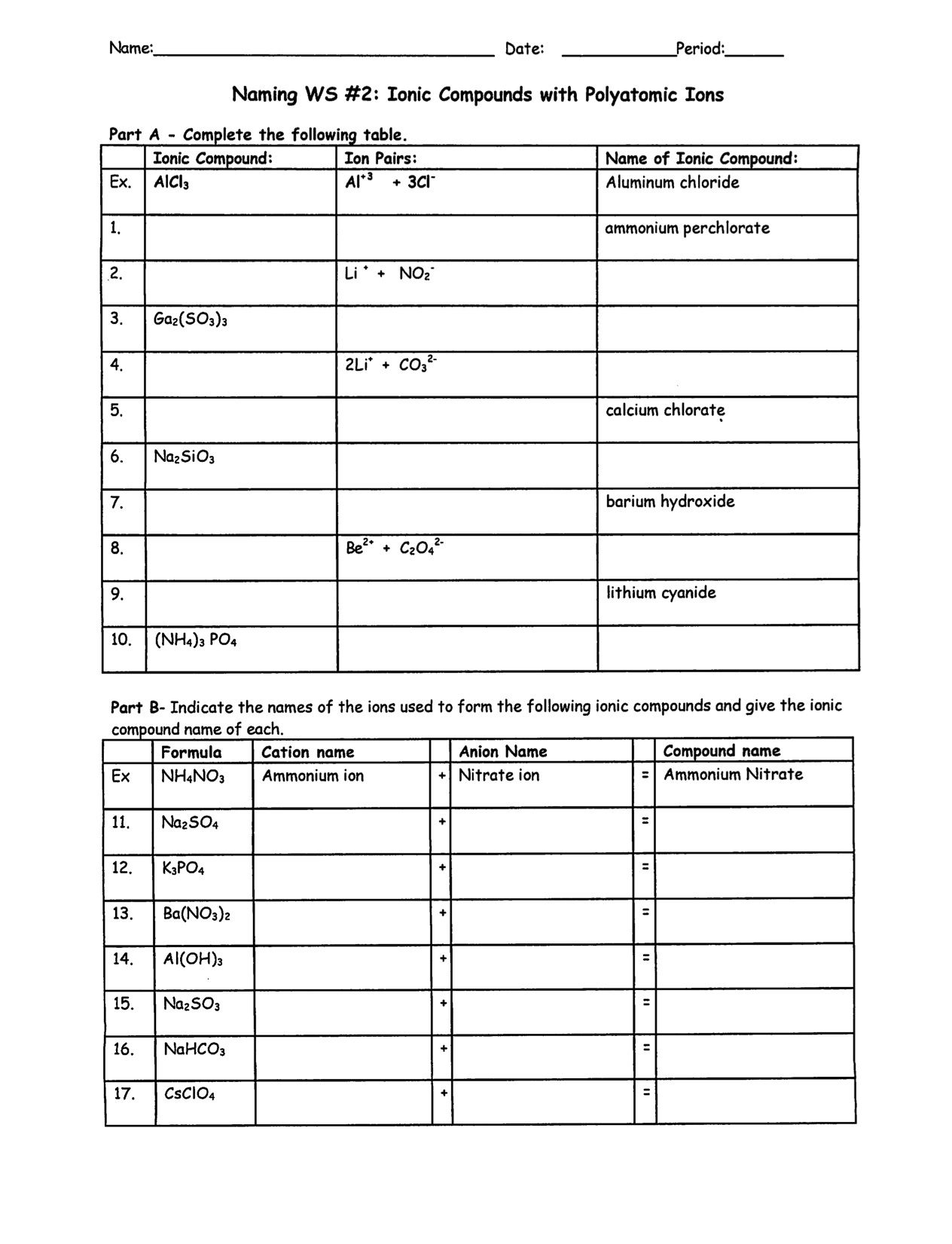
inspirevio.blogspot.com
Covalent Bonding Worksheet Teaching Resources – Vrogue.co

www.vrogue.co
Covalent Bonding Worksheet – Wendelina
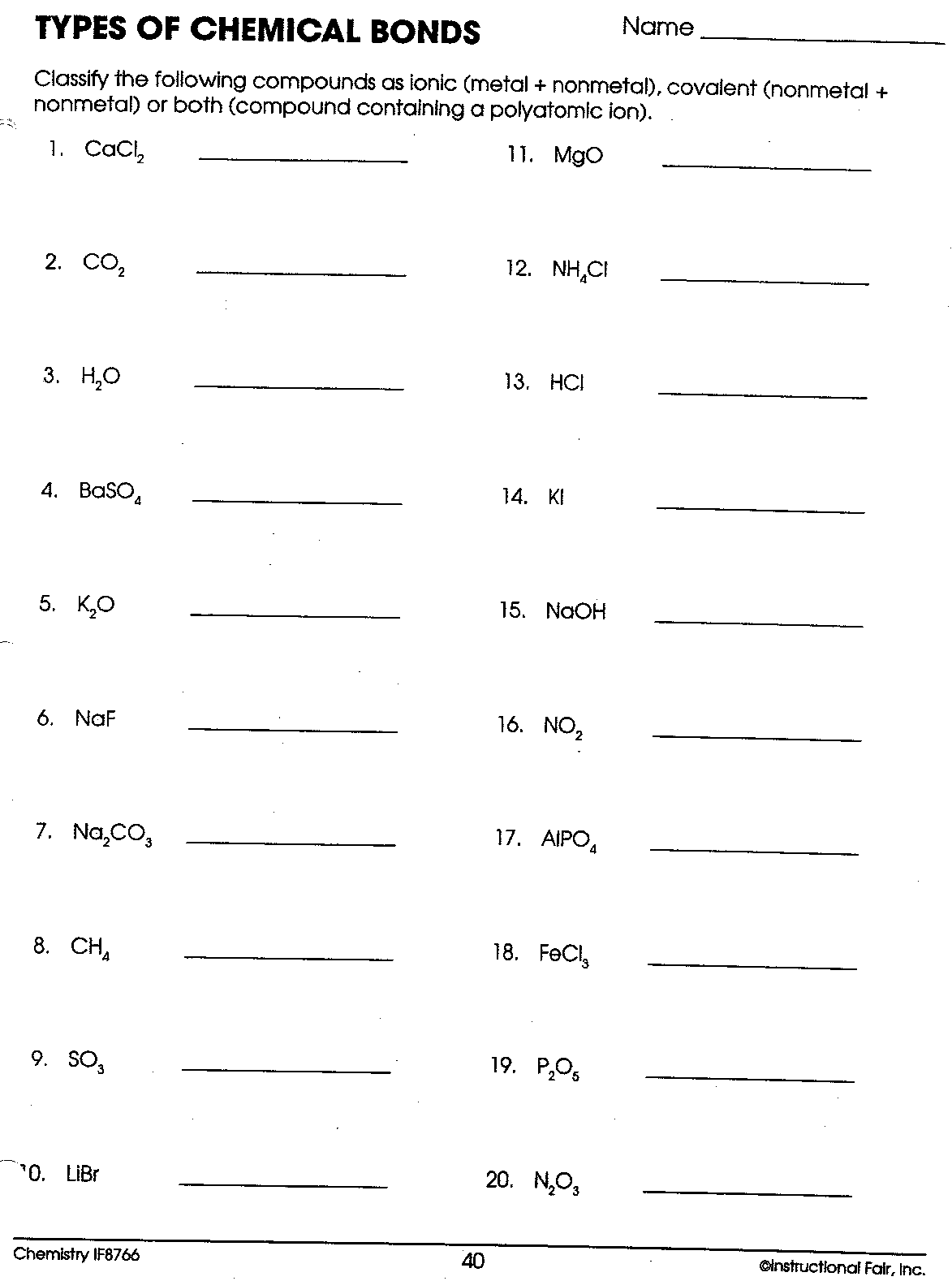
wendelina.blogspot.com
Covalent Bonding Worksheet Answer Key – Ame.my.id
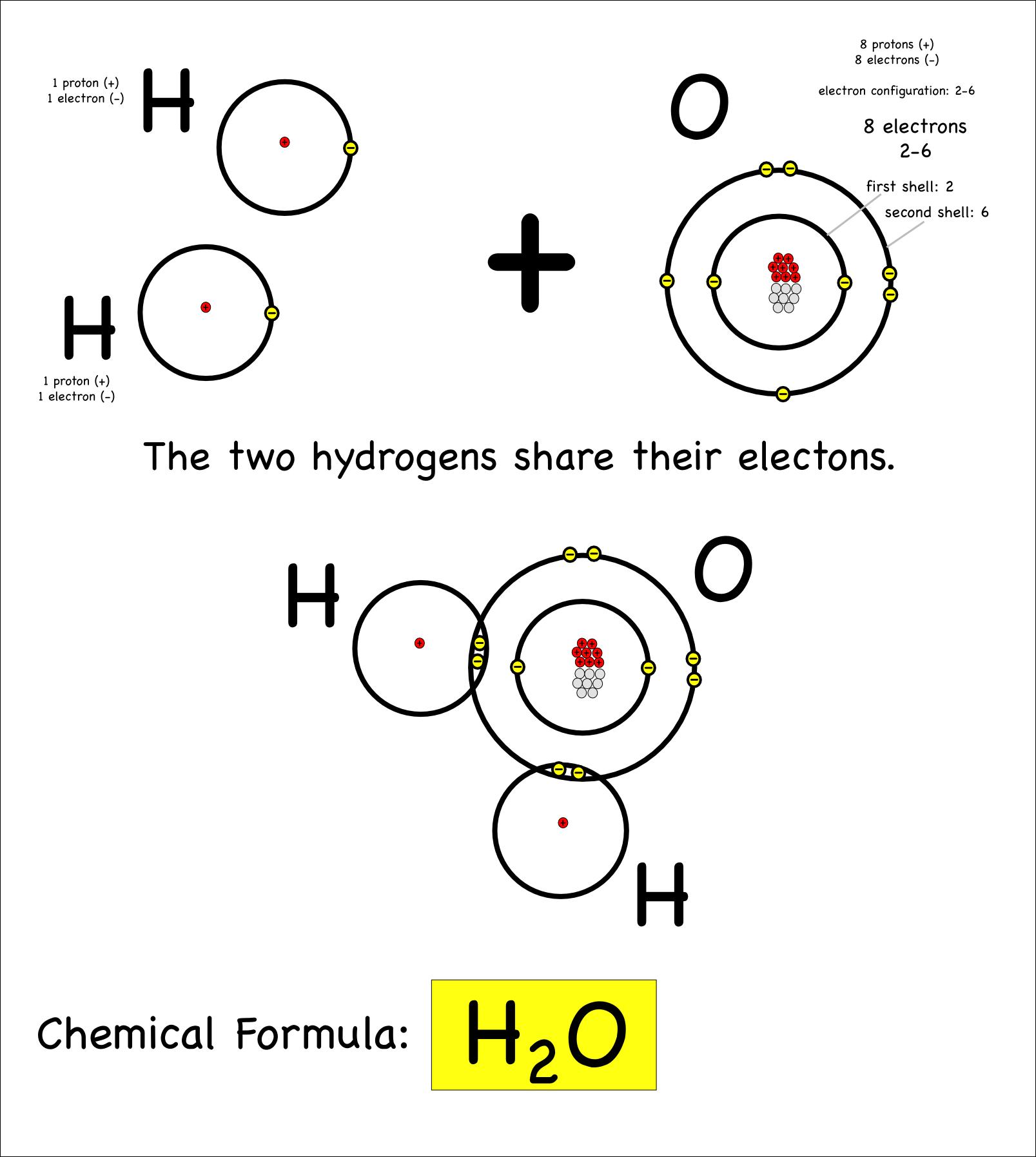
ame.my.id
Covalent Bonding Worksheet Answers – E-streetlight.com

www.e-streetlight.com
Understanding Covalent Bonding: Chapter 8 Worksheet Answers
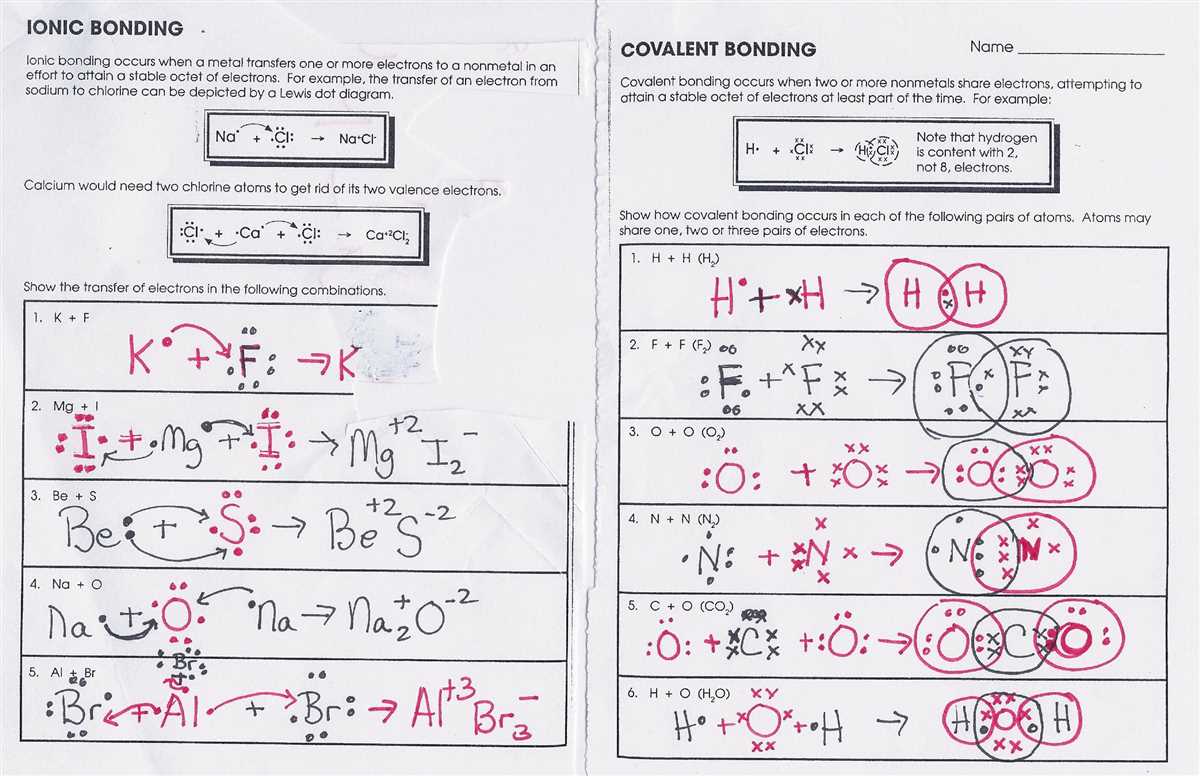
studyfinder.org
Covalent Bonding Worksheet Answers – E-streetlight.com
www.e-streetlight.com
covalent bonding worksheet teaching resources. Simple covalent bonding worksheets lewis electron dot structures …. covalent bonding worksheet