Struggling with Net Ionic Equations? You’re not alone! Many students find the transition from balancing regular chemical equations to understanding and writing net ionic equations a challenging hurdle in chemistry. This worksheet is designed to help you practice and master the process. We’ll guide you through the key concepts, including identifying strong electrolytes, weak electrolytes, and non-electrolytes, and writing complete ionic and net ionic equations. Remember, the goal is to focus only on the species that are actively participating in the reaction, eliminating those that are simply “spectators.” By understanding the “why” behind each step, you’ll move from rote memorization to genuine comprehension, allowing you to confidently tackle any net ionic equation problem.
Understanding Net Ionic Equations
A net ionic equation represents only the chemical species that are directly involved in a reaction. It focuses on the actual chemical change occurring in a solution. Before we dive into the worksheet answers, let’s quickly recap the key steps:
- Write the balanced molecular equation: This is the standard chemical equation, showing all reactants and products as neutral formulas. Make sure it’s balanced according to the law of conservation of mass.
- Write the complete ionic equation: Dissociate all strong electrolytes into their ions. Strong electrolytes include strong acids, strong bases, and soluble ionic compounds. Weak electrolytes and non-electrolytes remain as undissociated molecules. Remember to use solubility rules to determine if an ionic compound is soluble in water (aqueous).
- Identify spectator ions: These are ions that appear on both sides of the complete ionic equation, meaning they haven’t undergone any change during the reaction.
- Write the net ionic equation: Remove the spectator ions from the complete ionic equation. The remaining equation represents the net chemical change.
- Balance the net ionic equation: Ensure that both atoms and charges are balanced.
Common Mistakes to Avoid
- Forgetting to balance the initial molecular equation: An unbalanced molecular equation leads to an incorrect complete ionic and, consequently, an incorrect net ionic equation.
- Incorrectly dissociating compounds: Only strong electrolytes dissociate into ions. Weak electrolytes and non-electrolytes remain as molecules. Pay close attention to solubility rules!
- Failing to include states of matter: Always include the states of matter (aq, s, l, g) for each species in the equation.
- Not balancing the final net ionic equation: The net ionic equation must be balanced both in terms of atoms and charges.
- Confusing dissociation with dissolving: Dissolving is the process of a substance becoming dispersed in a solvent. Dissociation is the process of an ionic compound separating into its constituent ions when dissolved in a solvent. They are related but not the same.
Now, let’s move on to the answers to the Net Ionic Equations Worksheet. Remember to practice these problems and understand the reasoning behind each step. This understanding will be far more beneficial than simply memorizing the answers.
Net Ionic Equations Worksheet Answers
Below are the answers to a hypothetical Net Ionic Equations Worksheet. The specific reactions and their solutions would need to be determined based on the content of the actual worksheet you are using. However, I will provide examples that are representative of the type of problems typically found on such worksheets. Note that the “(aq)” and “(s)” notations are assumed for all ions and solids that are aqueous and solid, respectively.
-
Reaction: AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
- Complete Ionic Equation: Ag+(aq) + NO3–(aq) + Na+(aq) + Cl–(aq) → AgCl(s) + Na+(aq) + NO3–(aq)
- Net Ionic Equation: Ag+(aq) + Cl–(aq) → AgCl(s)
-
Reaction: H2SO4(aq) + 2NaOH(aq) → Na2SO4(aq) + 2H2O(l)
- Complete Ionic Equation: 2H+(aq) + SO42-(aq) + 2Na+(aq) + 2OH–(aq) → 2Na+(aq) + SO42-(aq) + 2H2O(l)
- Net Ionic Equation: 2H+(aq) + 2OH–(aq) → 2H2O(l) (Simplified: H+(aq) + OH–(aq) → H2O(l) )
-
Reaction: CuCl2(aq) + 2KOH(aq) → Cu(OH)2(s) + 2KCl(aq)
- Complete Ionic Equation: Cu2+(aq) + 2Cl–(aq) + 2K+(aq) + 2OH–(aq) → Cu(OH)2(s) + 2K+(aq) + 2Cl–(aq)
- Net Ionic Equation: Cu2+(aq) + 2OH–(aq) → Cu(OH)2(s)
-
Reaction: Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
- Complete Ionic Equation: Mg(s) + 2H+(aq) + 2Cl–(aq) → Mg2+(aq) + 2Cl–(aq) + H2(g)
- Net Ionic Equation: Mg(s) + 2H+(aq) → Mg2+(aq) + H2(g)
-
Reaction: Na2CO3(aq) + 2HCl(aq) → 2NaCl(aq) + H2O(l) + CO2(g)
- Complete Ionic Equation: 2Na+(aq) + CO32-(aq) + 2H+(aq) + 2Cl–(aq) → 2Na+(aq) + 2Cl–(aq) + H2O(l) + CO2(g)
- Net Ionic Equation: CO32-(aq) + 2H+(aq) → H2O(l) + CO2(g)
Remember to use these examples as a guide. The key to mastering net ionic equations is consistent practice and a solid understanding of solubility rules and the behavior of strong and weak electrolytes. Good luck!
If you are searching about Net Ionic Equation Worksheet | Exercises Chemistry | Docsity you’ve came to the right web. We have 20 Images about Net Ionic Equation Worksheet | Exercises Chemistry | Docsity like Net Ionic Equations Worksheet Fresh Quiz & Worksheet Chemical Equations, A Visual Introduction to Ionic and Net Ionic Equations – Carolina and also Chemistry Balancing Chemical Equations Worksheet. Read more:
Net Ionic Equation Worksheet | Exercises Chemistry | Docsity
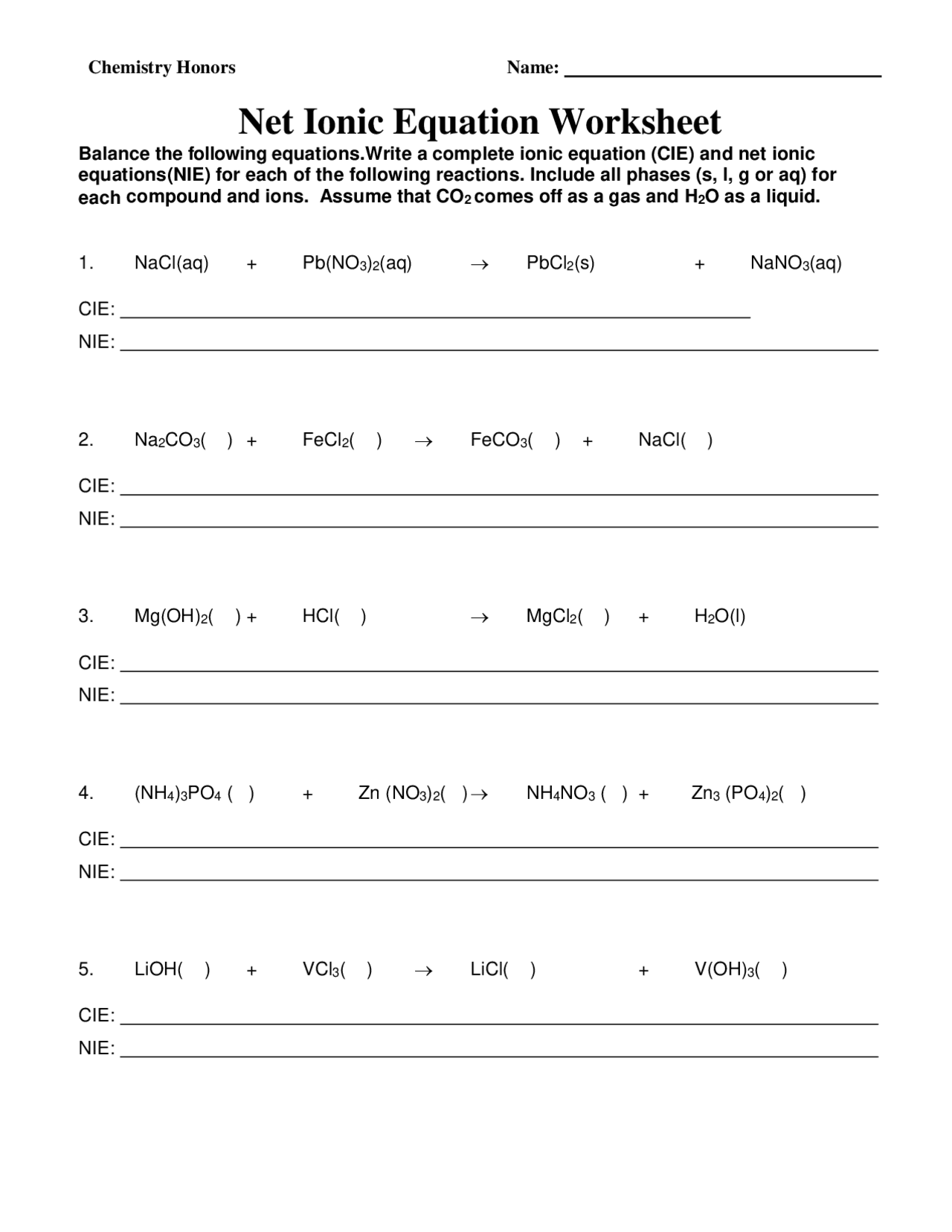
www.docsity.com
Net Ionic Equations Worksheet

www.proworksheet.my.id
Net Ionic Equations Worksheet – E-streetlight.com

www.e-streetlight.com
Net Ionic Equations Worksheet Answer Key – Printable PDF Template

martinlindelof.com
Ionic Equations Questions And Answers From Www.ChemistryTuition.Net

worksheets.clipart-library.com
Ionic Equations Worksheet And Answers – Printable Calendars AT A GLANCE

ataglance.randstad.com
NET Ionic Equations Worksheet – Studocu – Worksheets Library
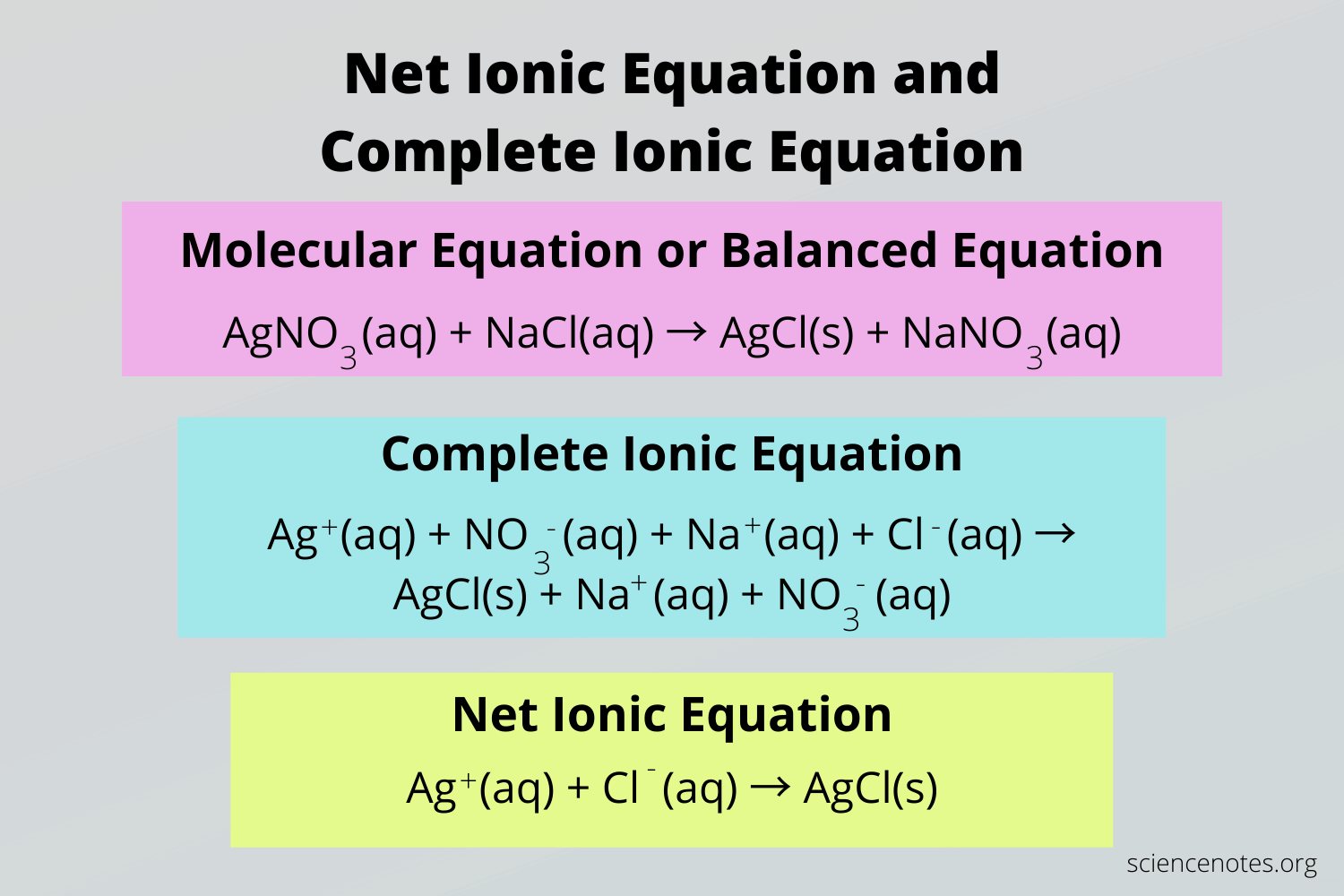
worksheets.clipart-library.com
Writing Ionic Equations Worksheet – Smarterinspire

smarterinspire.blogspot.com
How To Write Net Ionic Equations | Method + Examples

topblogtenz.com
Mastering Writing Net Ionic Equations For Precipitation Reactions: A

www.worksheetsdigital.co
Ionic Equations Worksheet – CHEM-155 – Studocu

www.studocu.com
Chemistry Balancing Chemical Equations Worksheet

worksheetdbmenses.z21.web.core.windows.net
Net Ionic Equation Worksheet Answers | Study Notes Chemistry | Docsity

worksheets.clipart-library.com
Naming Ionic Compounds Practice Worksheet | Lecture Notes

worksheets.clipart-library.com
Net Ionic Equations Worksheet Fresh Quiz & Worksheet Chemical Equations

chessmuseum.org
Net Ionic Equations Worksheet – E-streetlight.com

www.e-streetlight.com
Net Ionic Equations Worksheet

www.proworksheet.my.id
Worksheet – Net Ionic Equations – Worksheets Library

worksheets.clipart-library.com
A Visual Introduction To Ionic And Net Ionic Equations – Carolina

worksheets.clipart-library.com
SOLUTION: Balancing Equations Worksheet – Studypool – Worksheets Library

worksheets.clipart-library.com
Chemistry balancing chemical equations worksheet. How to write net ionic equations. Ionic equations questions and answers from www.chemistrytuition.net