Photoelectron Spectroscopy (PES), also known as XPS (X-ray Photoelectron Spectroscopy) or ESCA (Electron Spectroscopy for Chemical Analysis), is a powerful surface-sensitive quantitative spectroscopic technique used to determine the elemental composition, empirical formula, chemical state, and electronic state of the elements within a material. It achieves this by irradiating a material with a beam of X-rays and simultaneously measuring the kinetic energy and number of electrons that escape from the material. The PES spectrum, a plot of the number of electrons detected versus their binding energy, provides a unique fingerprint of the elements present and their chemical environment. Because PES is so sensitive to surface chemistry (only probing the top few nanometers), it’s widely used in materials science, chemistry, physics, and engineering for characterizing thin films, catalysts, polymers, semiconductors, and many other materials.
Understanding PES spectra requires careful interpretation of the binding energy values and peak intensities. The binding energy is characteristic of a particular element and its chemical state, while the peak intensity is related to the concentration of that element. Students often grapple with interpreting these spectra and applying theoretical principles to experimental data. That’s why a well-designed PES worksheet is crucial for reinforcing understanding and building problem-solving skills.
This post provides answers to a hypothetical PES worksheet, along with explanations to help clarify the concepts involved. Remember that real-world spectra can be more complex, and a thorough understanding of the instrument parameters, sample preparation, and data processing techniques is essential for accurate analysis.
Photoelectron Spectroscopy Worksheet Answers
Below are the answers to a hypothetical PES worksheet. The questions are implied by the answer format. Each answer is presented with explanations and considerations to help you understand the underlying principles.
Question 1: Identifying Elements from Binding Energies
- Answer:
Element A: Oxygen (O 1s ~ 532 eV)Explanation: The binding energy of approximately 532 eV is characteristic of the O 1s core level, which is commonly observed in oxides, hydroxides, and organic compounds containing oxygen. Variations can occur depending on the specific chemical environment.
- Answer:
Element B: Carbon (C 1s ~ 285 eV)Explanation: A binding energy around 285 eV is typically associated with the C 1s core level. This is a common element in most samples, and variations in the C 1s peak can provide information about different carbon bonding environments, such as C-C, C-O, and C=O bonds. The exact value can shift based on the bonding partner and level of oxidation.
- Answer:
Element C: Gold (Au 4f7/2 ~ 84 eV)Explanation: The Au 4f7/2 core level exhibits a characteristic binding energy of approximately 84 eV for metallic gold. This peak is often used for calibration purposes due to its well-defined position. Remember the spin-orbit splitting, you should also see Au 4f5/2 peak at higher binding energy.
Question 2: Determining Elemental Composition
- Answer:
Atomic Ratio: O:C = 1:2Explanation: This is determined by comparing the areas under the O 1s and C 1s peaks after applying appropriate sensitivity factors (also known as atomic sensitivity factors or ASF). The formula is (Area O 1s / ASF O 1s) : (Area C 1s / ASF C 1s). Sensitivity factors are element-specific and take into account the probability of photoemission. You’ll need to look up or be provided with those factors to calculate this correctly. Once you calculate both ratios, divide by the smallest value to get a simplest whole number ratio.
Question 3: Identifying Chemical States
- Answer:
Two oxidation states of carbon are present: C-C/C-H (285.0 eV) and C-O (286.5 eV).Explanation: Deconvolution of the C 1s peak reveals two components. The lower binding energy component (285.0 eV) corresponds to sp3 hybridized carbon, characteristic of C-C or C-H bonds. The higher binding energy component (286.5 eV) indicates carbon bonded to oxygen, such as in an alcohol or ether functional group. Proper curve fitting with appropriate peak shapes (usually Gaussian-Lorentzian mixes) and constraints (e.g., fixed full width at half maximum, FWHM) is crucial for accurate deconvolution.
Question 4: Analyzing Spectral Features
- Answer:
The presence of a satellite peak at higher binding energy to the main core level peak suggests a shake-up transition.Explanation: Satellite peaks, also known as shake-up or shake-off satellites, are weaker peaks that appear at higher binding energies relative to the main core level peak. They arise from electronic excitations that occur simultaneously with the photoemission process. Specifically, a “shake-up” transition involves an electron being excited to a higher, unoccupied energy level. Their presence can provide additional information about the electronic structure and bonding characteristics of the material.
Question 5: Applications of PES
- Answer:
PES can be used to study surface oxidation, identify contaminants, and characterize thin films.Explanation: PES’s surface sensitivity makes it ideal for studying surface phenomena like oxidation or corrosion. It can identify the presence and concentration of surface contaminants. Furthermore, its ability to probe the chemical composition and electronic structure of thin films makes it essential for characterizing these materials, which are widely used in various technological applications.
This hypothetical worksheet provides a glimpse into the power of PES for materials characterization. Remember to always consider the specific details of your sample and experimental setup for accurate interpretation of PES spectra. Further research into specific elements and their binding energies is often needed for more complex samples.
If you are searching about Photoelectron Spectroscopy Worksheet Answers – Englishworksheet.my.id you’ve visit to the right web. We have 20 Pictures about Photoelectron Spectroscopy Worksheet Answers – Englishworksheet.my.id like Photoelectron Spectroscopy Worksheet Answers – Englishworksheet.my.id, Photoelectron spectroscopy worksheet answers worksheet for education and also Solved Spectroscopy Worksheet Chemistry 2540 4. Please | Chegg.com. Read more:
Photoelectron Spectroscopy Worksheet Answers – Englishworksheet.my.id
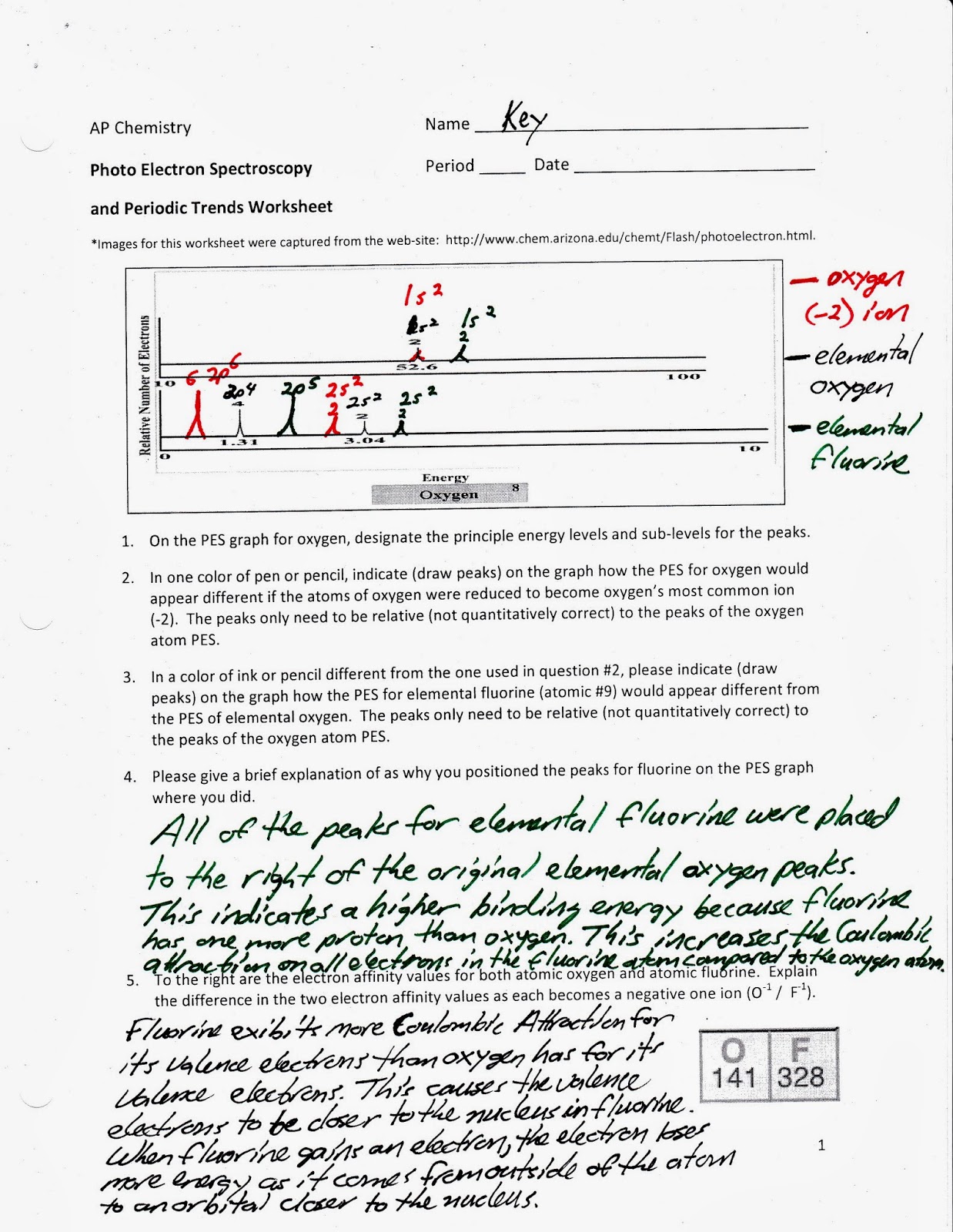
www.englishworksheet.my.id
UV-visible Spectroscopy Worksheet.doc
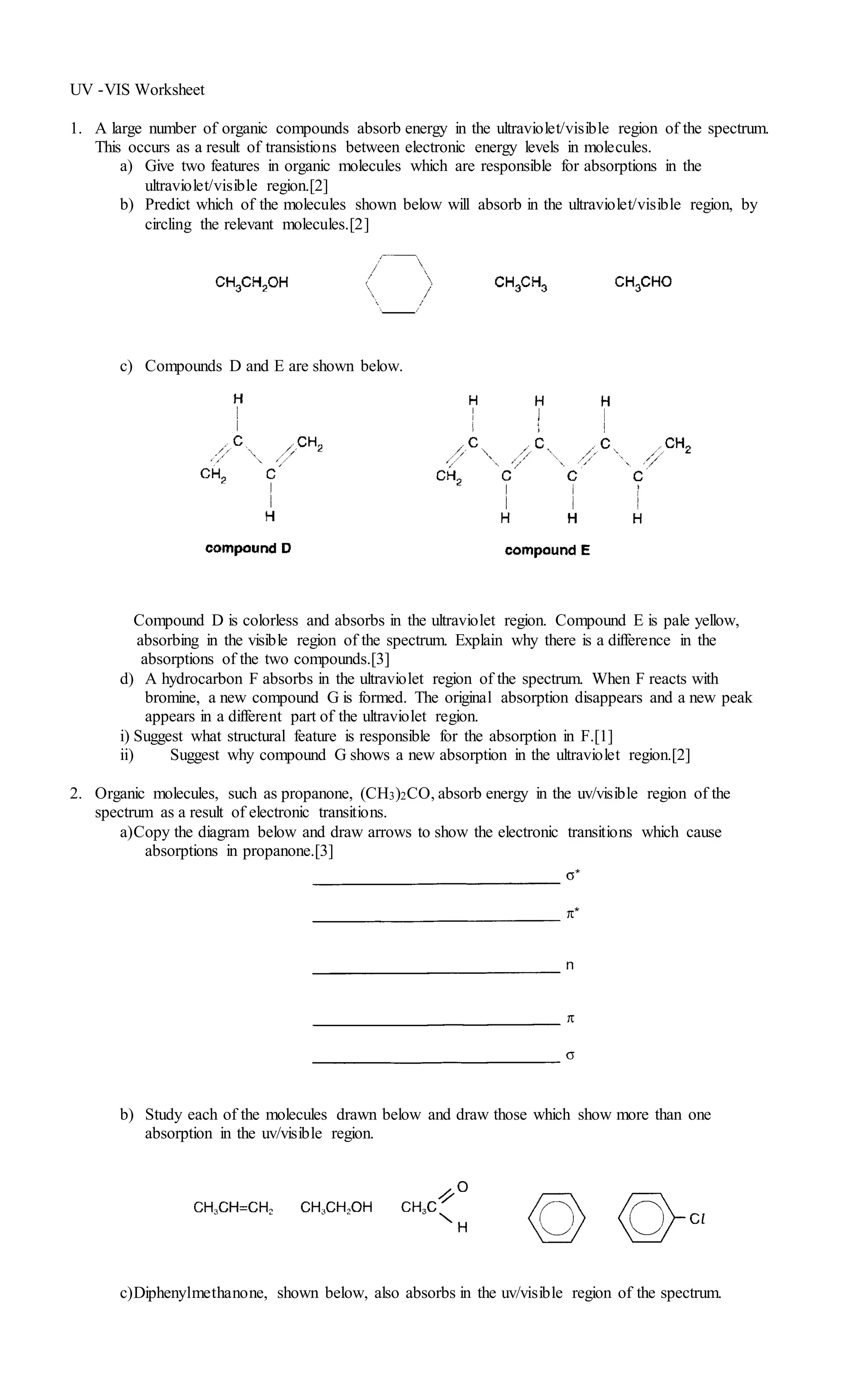
www.slideshare.net
IR Spectroscopy Worksheet With Answers – Chem241 (CHEM241) – Stuvia US

www.stuvia.com
SOLUTION: Lower Secondary Science 8 Worksheet Answers – Cambridge

worksheets.clipart-library.com
Photoelectron Spectroscopy Worksheet Answers – Ame.my.id
ame.my.id
SOLUTION: Infrared Spectroscopy Worksheet – Studypool

www.studypool.com
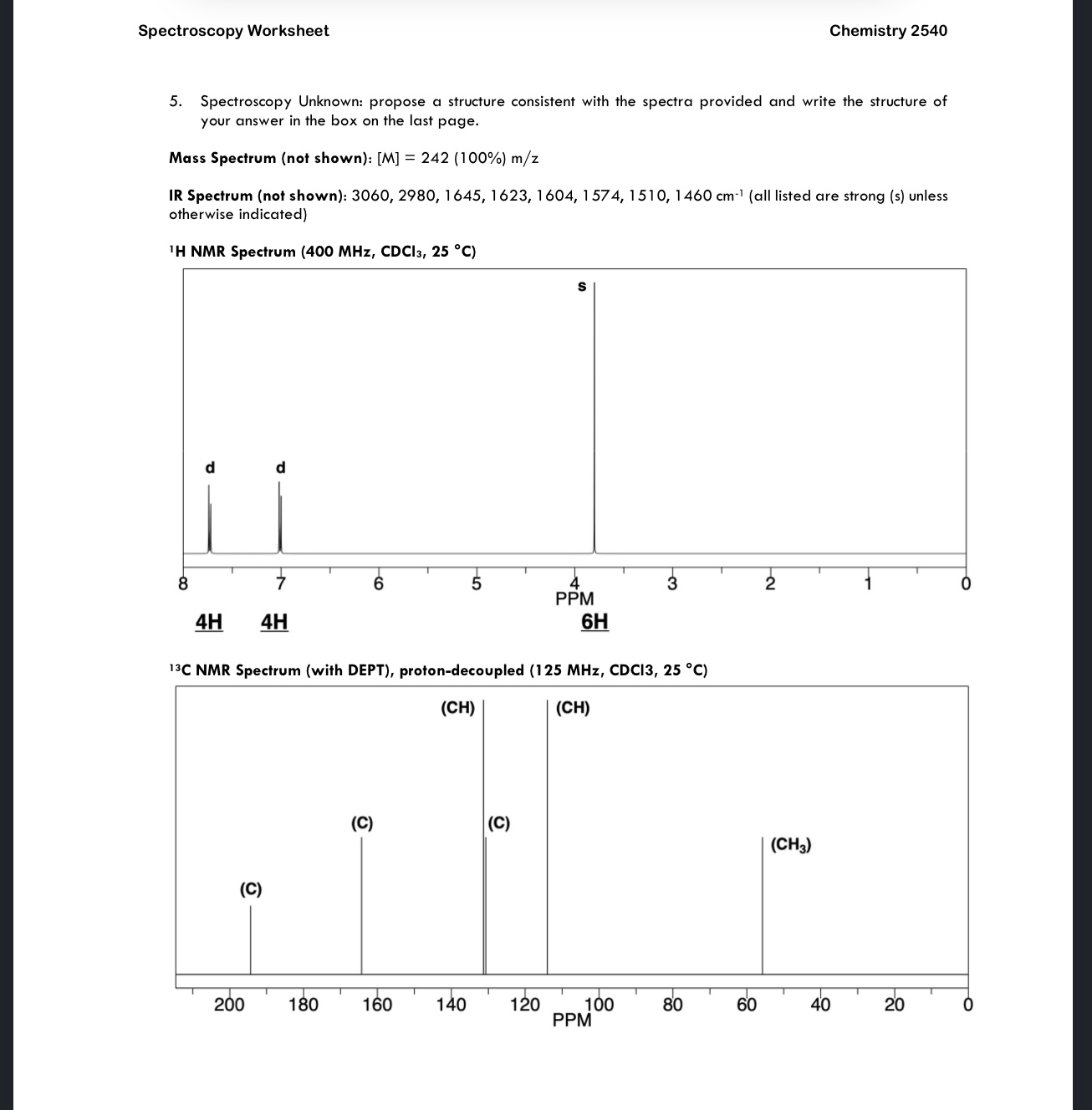
Solved Spectroscopy Worksheet Chemistry 2540 4. Please | Chegg.com
www.chegg.com
Photoelectron Spectroscopy Pes Worksheet Answers

materialcampushearsays.z21.web.core.windows.net
Worksheet Photoelectron Spectroscopy & Electron Configuration

davida.davivienda.com
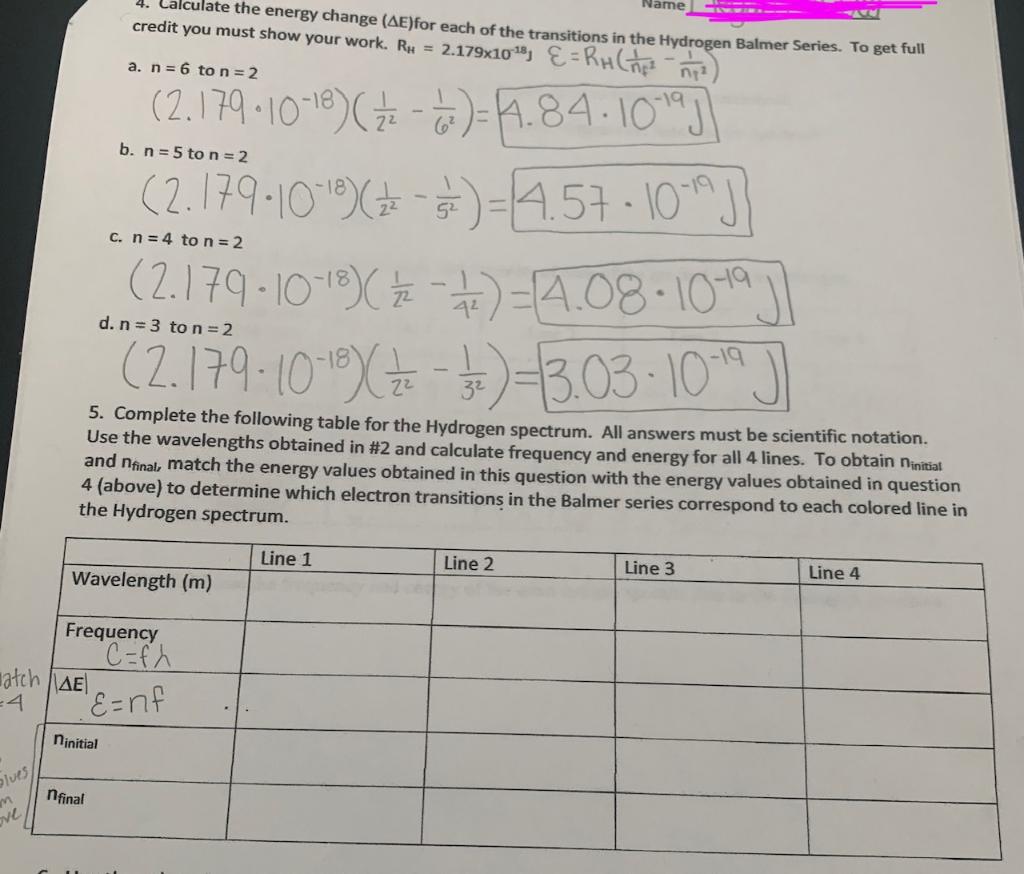
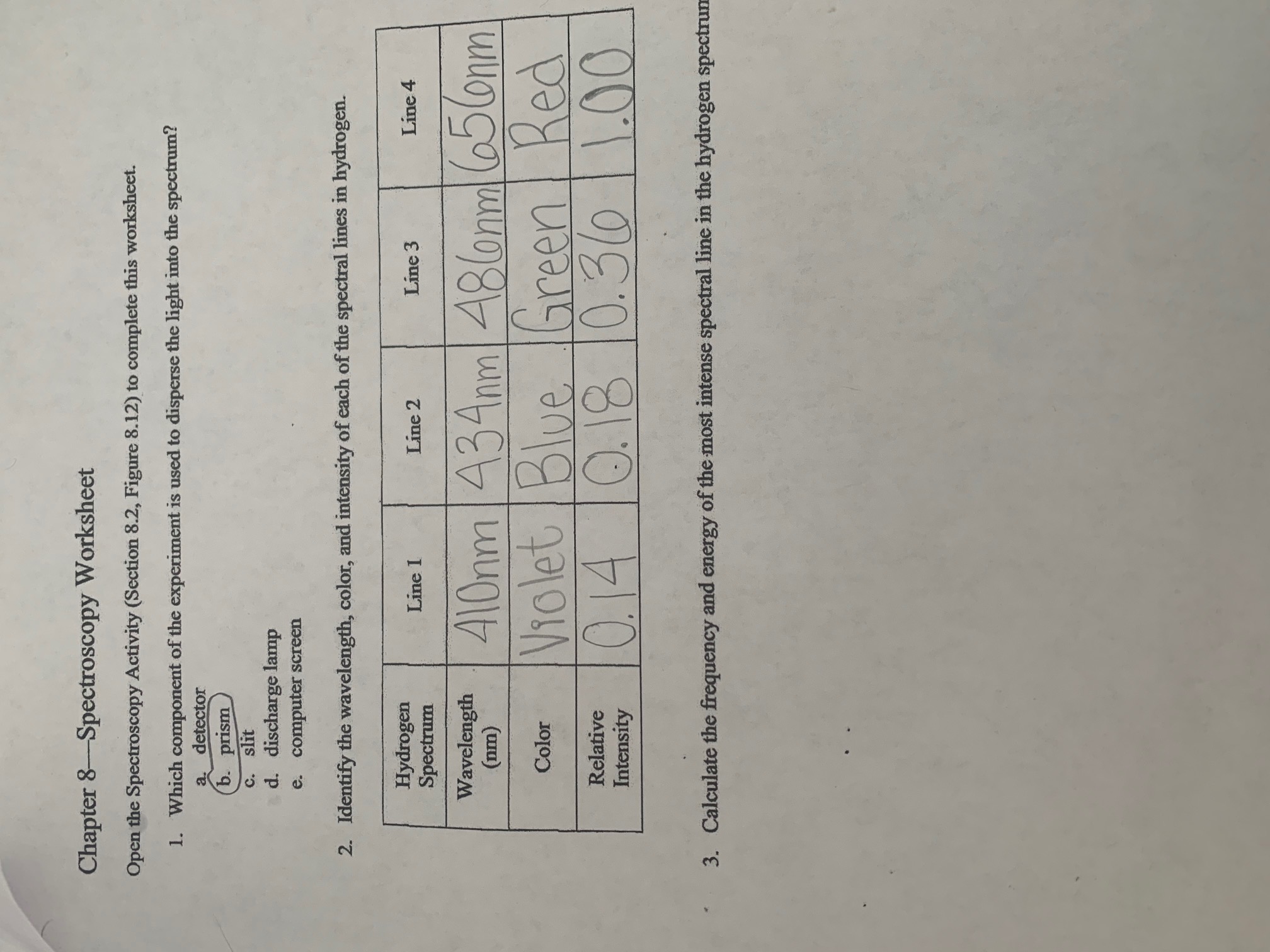
Solved Chapter 8—Spectroscopy Worksheet Open The | Chegg.com
www.chegg.com
The Electromagnetic Spectrum Worksheet Answers 33 Waves Worksheet

www.pinterest.com
Photoelectron Spectroscopy Worksheet Answers Worksheet For Education

www.artofit.org
Ap Chemistry Photoelectron Spectroscopy Worksheet — Db-excel.com

db-excel.com
Solved Chapter 8—Spectroscopy Worksheet Open The | Chegg.com
www.chegg.com
Photoelectron Spectroscopy Worksheet Answers – Englishworksheet.my.id
www.englishworksheet.my.id
Photoelectron Spectroscopy Problems At Mae Kimbrell Blog
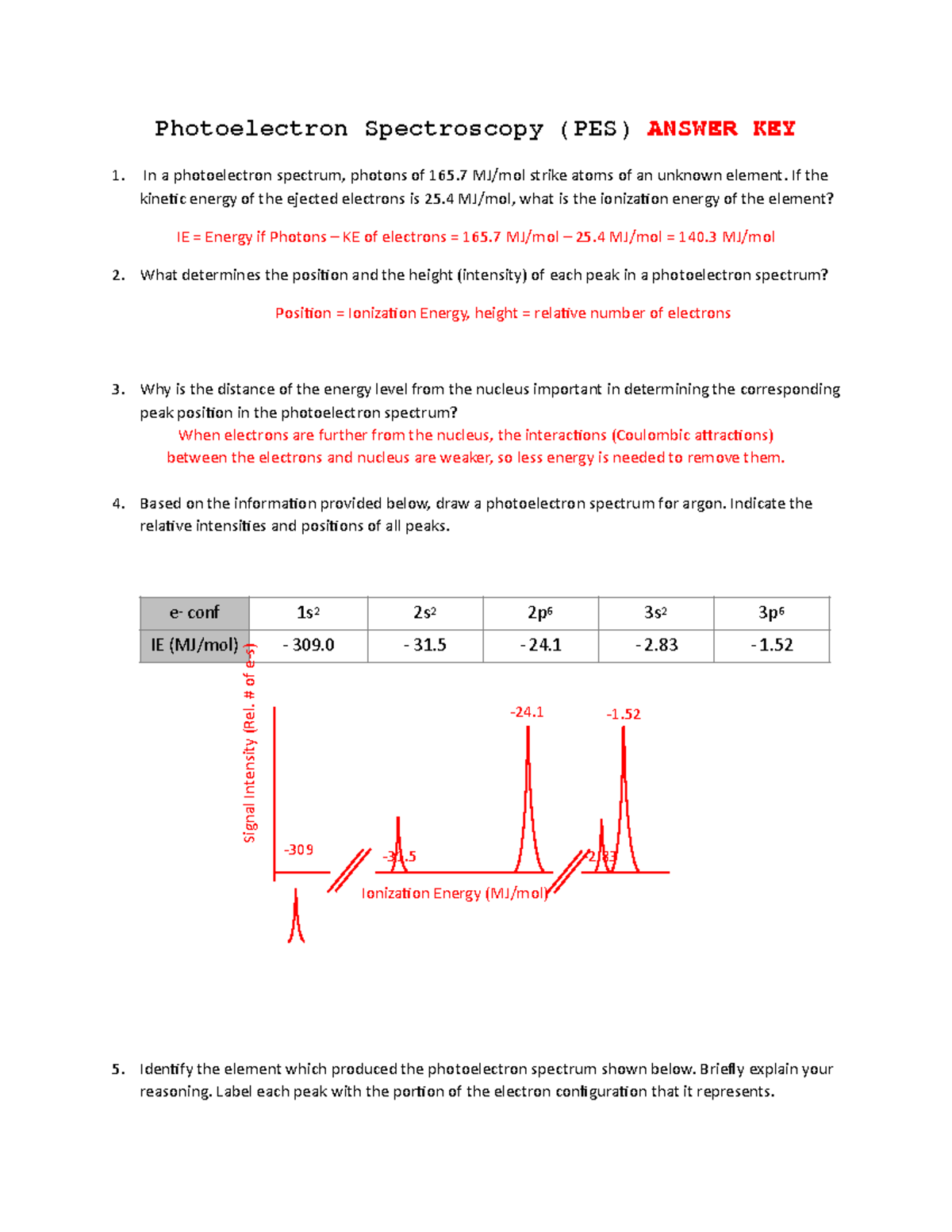
storage.googleapis.com
CC5758D – Photoelectron S Photoelectron Spectroscopy Worksheet-KEY

www.studocu.com
UV-visible Spectroscopy Worksheet.doc
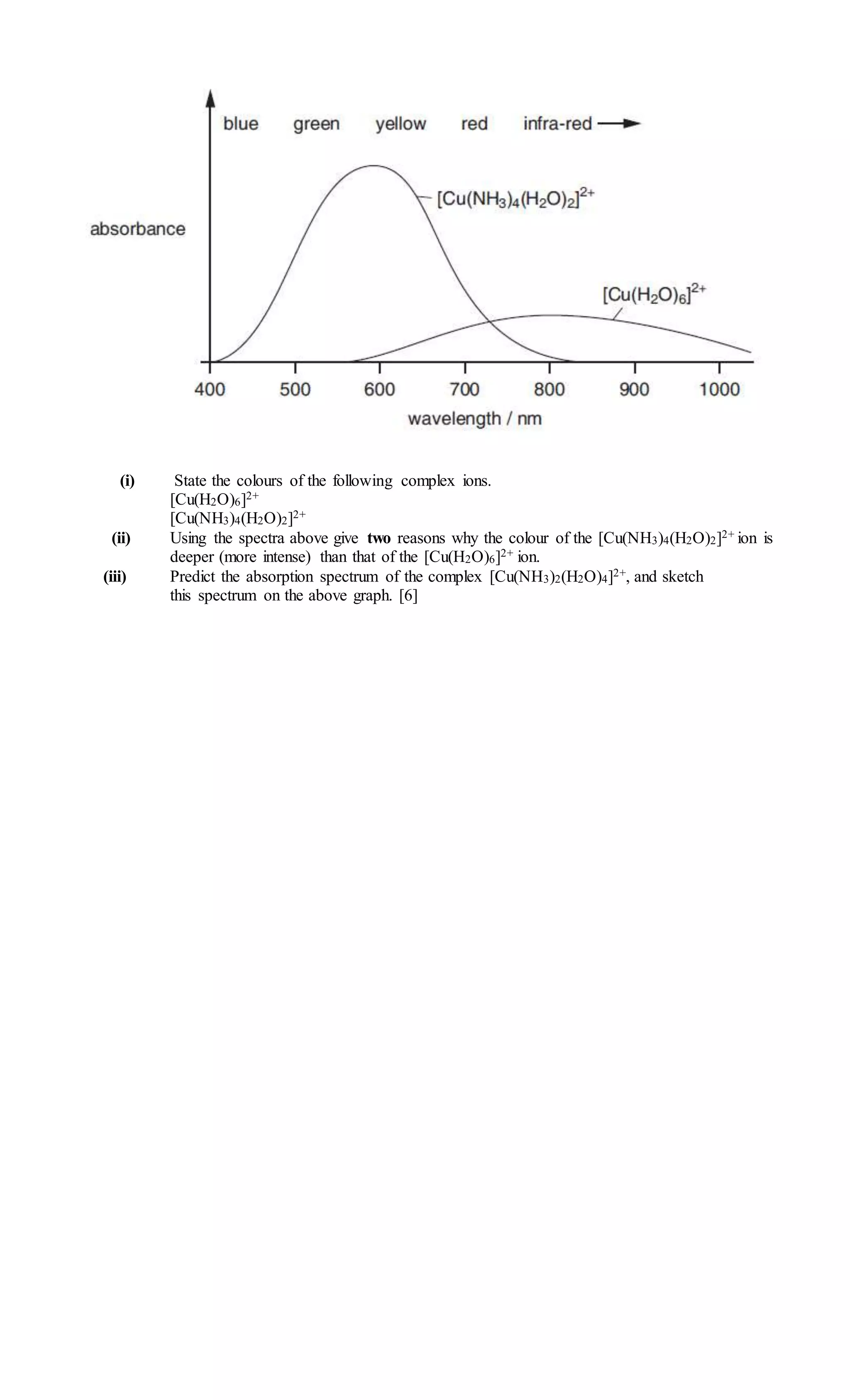
www.slideshare.net
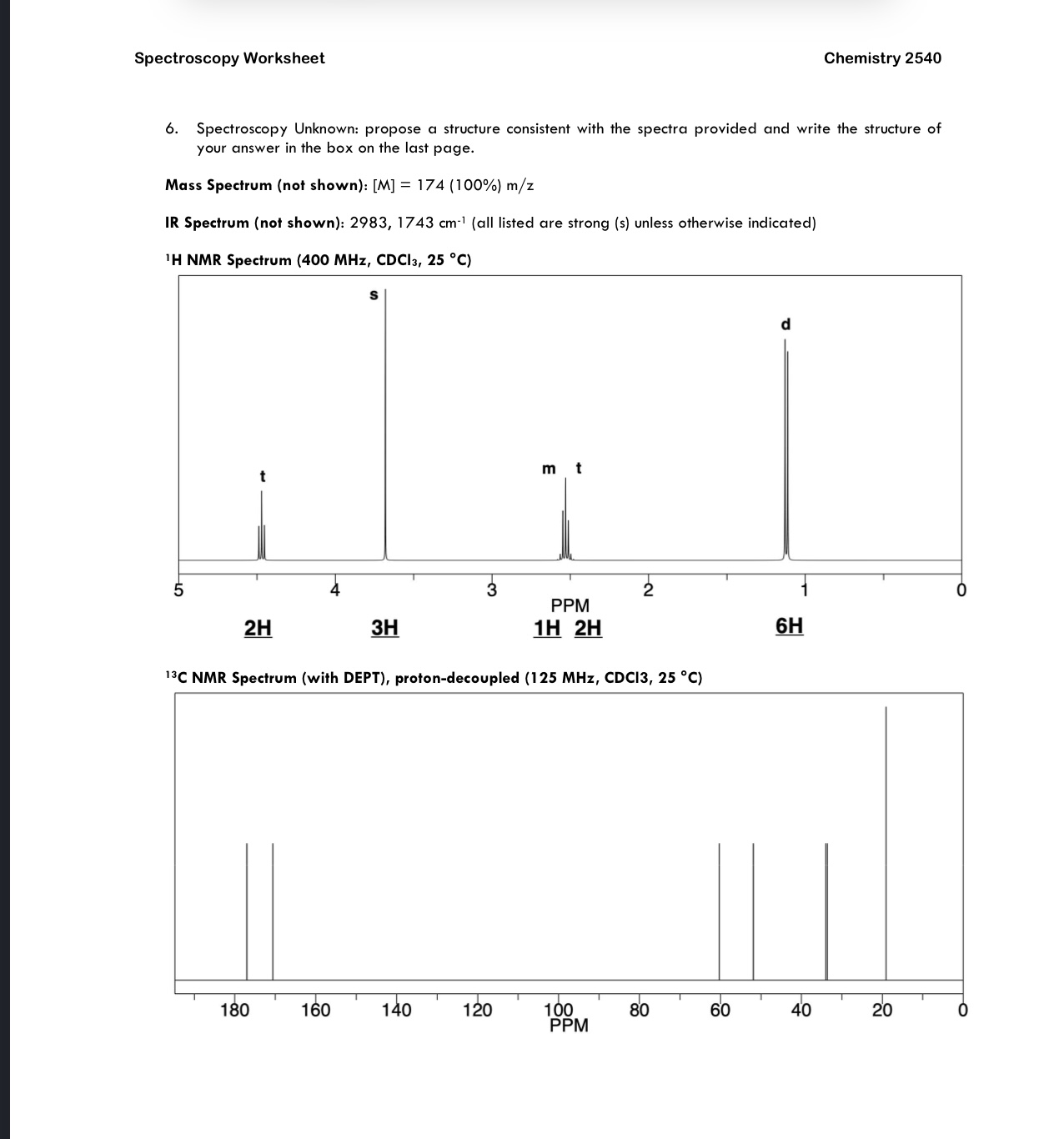
Solved Spectroscopy Worksheet Chemistry 2540 4. Please | Chegg.com
www.chegg.com
Photoelectron Spectroscopy Problems At Mae Kimbrell Blog
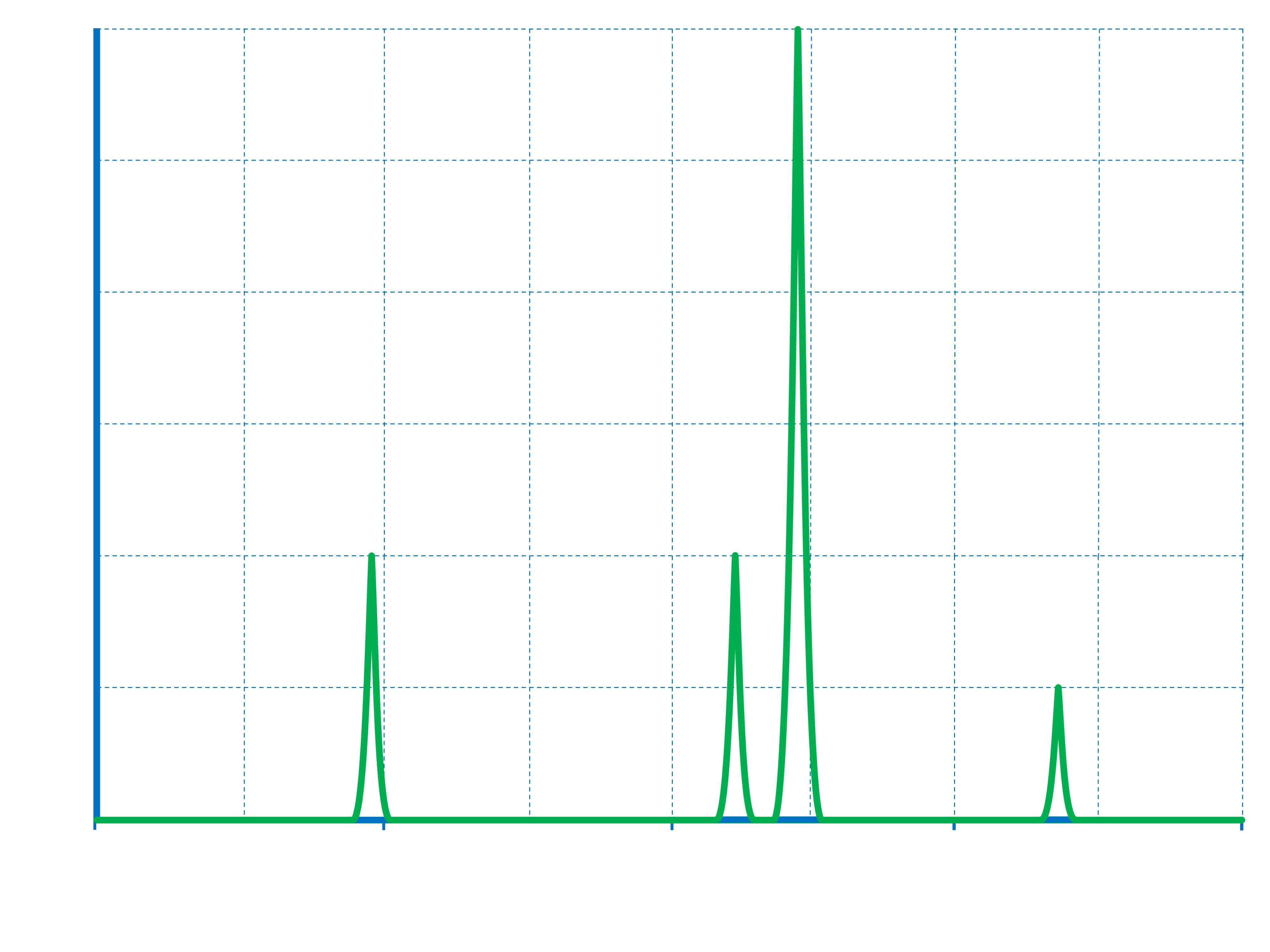
storage.googleapis.com
The electromagnetic spectrum worksheet answers 33 waves worksheet …. photoelectron spectroscopy worksheet answers. Worksheet photoelectron spectroscopy & electron configuration