Having trouble with your Boyle’s Law worksheet? You’re not alone! Many students find gas laws a bit tricky at first. Understanding the inverse relationship between pressure and volume at constant temperature can be a hurdle. This post aims to provide you with the answers to a typical Boyle’s Law worksheet and, more importantly, to explain the reasoning behind each solution. Remember, simply memorizing answers won’t help you in the long run. Focusing on grasping the underlying concepts is key to mastering this fundamental chemistry principle. This will not only help you on future quizzes and exams but also give you a solid foundation for understanding more complex gas laws. We’ll break down the problems step-by-step, showing you how to apply the formula, convert units (if necessary), and arrive at the correct answer. So, let’s dive in and conquer Boyle’s Law together!
Understanding Boyle’s Law
Boyle’s Law states that for a fixed amount of gas at constant temperature, the pressure and volume are inversely proportional. This means that as the pressure increases, the volume decreases, and vice versa. Mathematically, this is expressed as:
P1V1 = P2V2
Where:
- P1 = Initial pressure
- V1 = Initial volume
- P2 = Final pressure
- V2 = Final volume
It’s crucial to remember that the units for pressure and volume must be consistent on both sides of the equation. For example, if P1 is in atmospheres (atm), then P2 must also be in atmospheres. Similarly, if V1 is in liters (L), then V2 must also be in liters. If the problem gives you different units, you’ll need to convert them before applying the formula.
Common Challenges and How to Overcome Them
One of the most common mistakes students make is forgetting to convert units. Always double-check the units given in the problem and ensure they are consistent before plugging the values into the equation. Another challenge is identifying which values represent P1, V1, P2, and V2. Read the problem carefully and pay attention to the wording. Look for clues such as “initial,” “final,” “changed to,” or “increased to” to help you identify the correct values.
Practice is also key! The more problems you solve, the more comfortable you’ll become with applying Boyle’s Law. Don’t be afraid to ask for help from your teacher or classmates if you’re struggling. Working through problems together can be a great way to solidify your understanding.
Boyle’s Law Worksheet Answers
Below are the answers to some typical Boyle’s Law problems. Remember to review the explanations to understand *why* these are the correct answers. While these are presented here, the process is important, so make sure you practice other Boyle’s Law problems on your own too.
- Problem 1: A gas occupies a volume of 10.0 L at standard pressure (1 atm). What volume will it occupy if the pressure is increased to 2.5 atm, assuming the temperature remains constant?
- Answer: V2 = 4.0 L
- Problem 2: A balloon contains 30 L of air at 27°C and 1 atm. If the pressure is doubled, what will be the new volume of the balloon?
- Answer: V2 = 15.0 L
- Problem 3: A 5.0 L flask contains gas at a pressure of 100 kPa. If the gas is allowed to expand to a volume of 15.0 L, what will be the new pressure?
- Answer: P2 = 33.3 kPa
- Problem 4: A gas with a volume of 4.0 L at a pressure of 205 kPa is allowed to expand to a volume of 12.0 L. What is the pressure in the container if the temperature remains constant?
- Answer: P2 = 68.3 kPa
- Problem 5: If the pressure of a gas is changed from 760 mm Hg to 380 mm Hg, what will be the new volume if the original volume was 150.0 cm3?
- Answer: V2 = 300.0 cm3
Remember that understanding the process of applying the formula and performing unit conversions (if needed) is more important than simply knowing the answers. Practice makes perfect!
If you are searching about Ideal Gas Law Worksheet – Ideal Gas Law Practice Worksheet Solve the you’ve visit to the right place. We have 20 Pics about Ideal Gas Law Worksheet – Ideal Gas Law Practice Worksheet Solve the like 9-14a,b Boyles Law and Charless Law wkst-Key – Worksheet: Boyles, SOLUTION: Ideal gas law worksheet 2 answer – Studypool – Worksheets Library and also SOLUTION: Ideal gass law worksheet – Studypool – Worksheets Library. Here you go:
Ideal Gas Law Worksheet – Ideal Gas Law Practice Worksheet Solve The

www.studocu.com
Science 9 Ohm's Law Worksheet Answers – Scienceworksheets.net
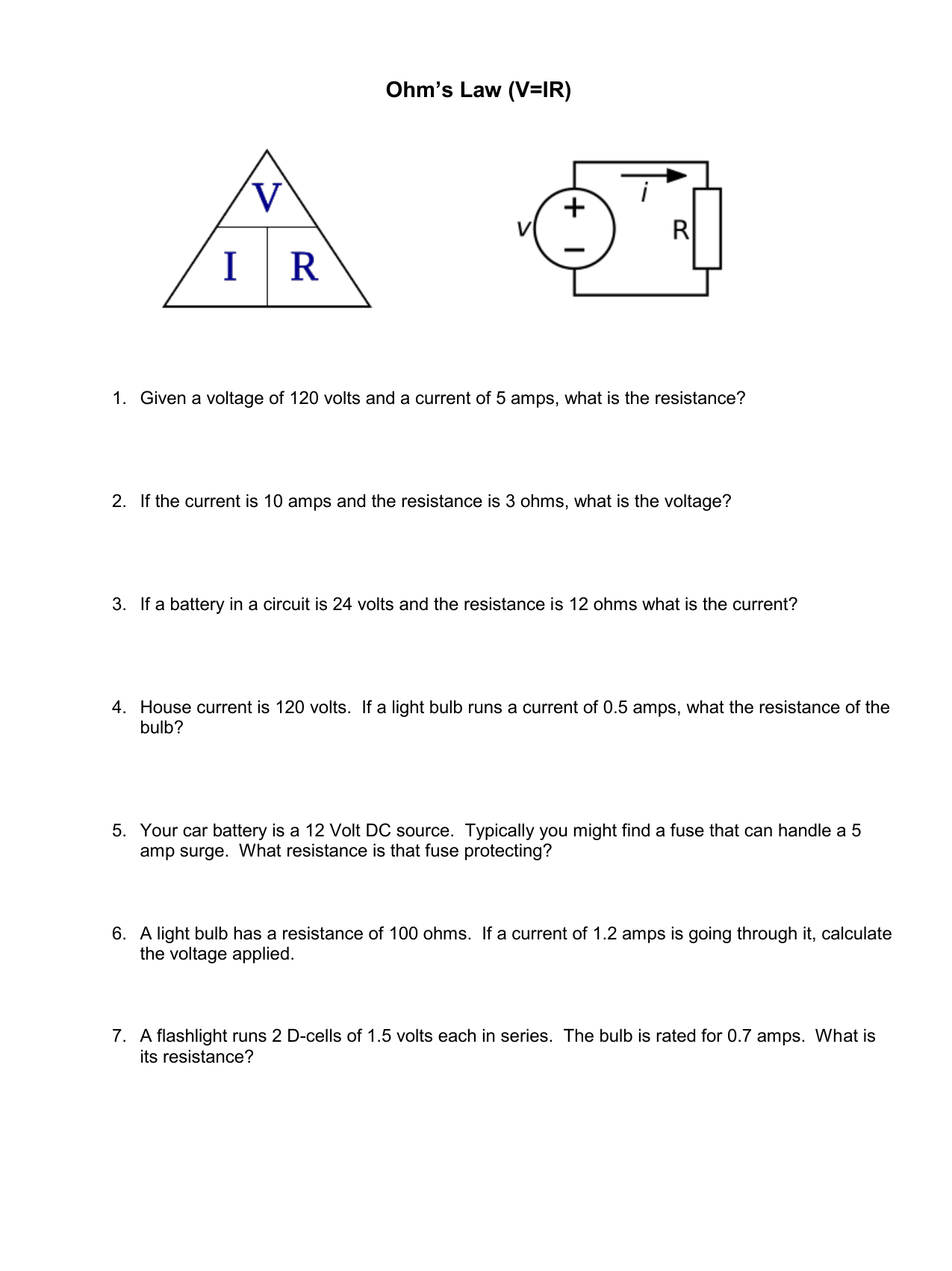
www.scienceworksheets.net
Boyle21s Law Worksheet Answer Key – Owhentheyanks.com

www.owhentheyanks.com
Boyle S Law Worksheet Answers – Worksheets For Kindergarten

worksheets.ekocraft-appleleaf.com
Newton Laws Worksheet Answers – E-streetlight.com
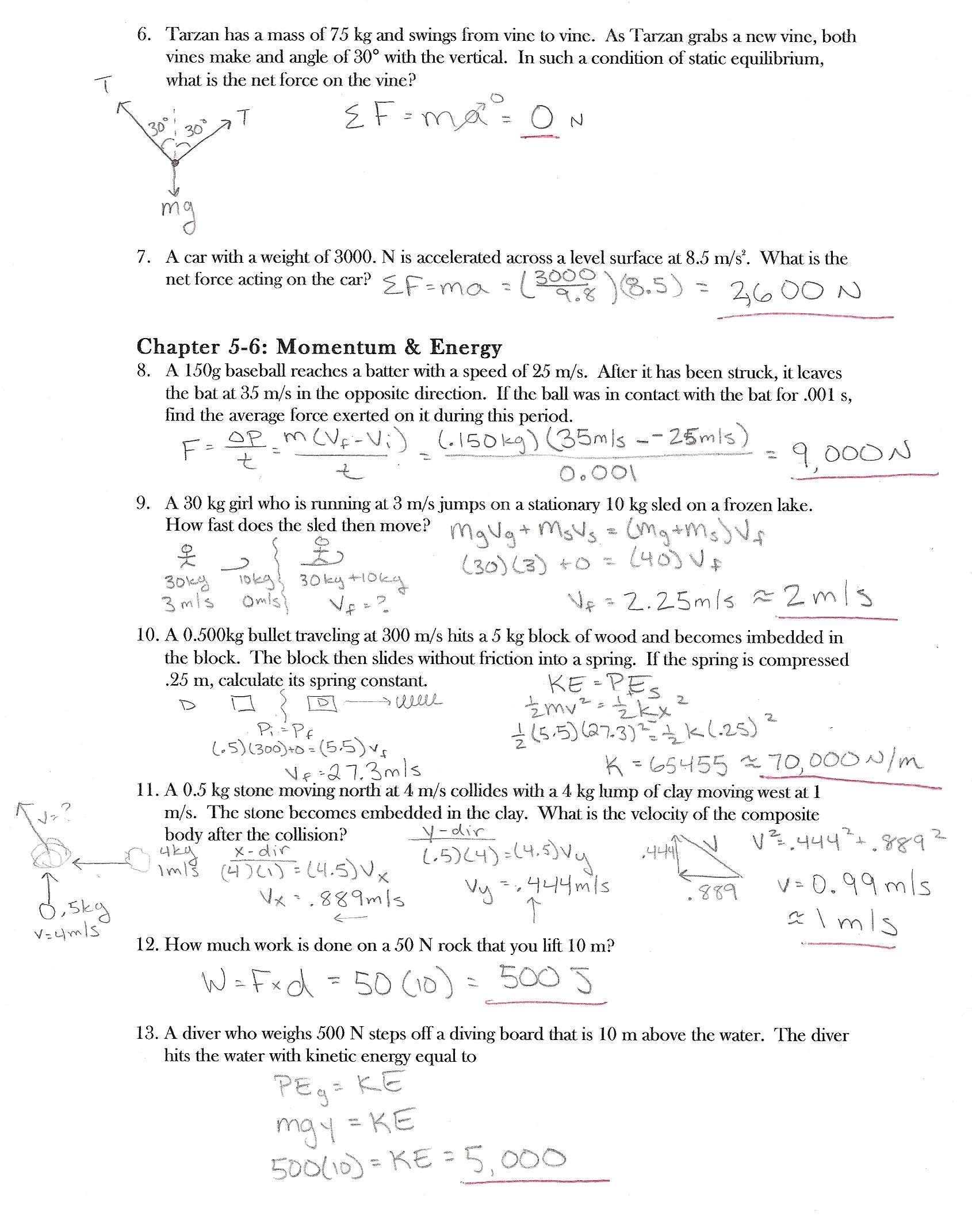
www.e-streetlight.com
Diysity: Boyle's Law Worksheet Answers Chapter 16

diysity.blogspot.com
Boyles Law And Charles Law Worksheet – CHE 321 – MSU – Studocu

www.studocu.com
Mixed Gas Laws Worksheet – CHEM-155 – Studocu – Worksheets Library

worksheets.clipart-library.com
Master Boyle's Law Practice Problems With Detailed Answers

education2research.com
Charles Law Worksheet Answers – Pro Worksheet

www.proworksheet.my.id
Boyle22s Law Worksheet Answers – E-streetlight.com

www.e-streetlight.com
SOLUTION: Ideal Gas Law Worksheet 2 Answer – Studypool – Worksheets Library
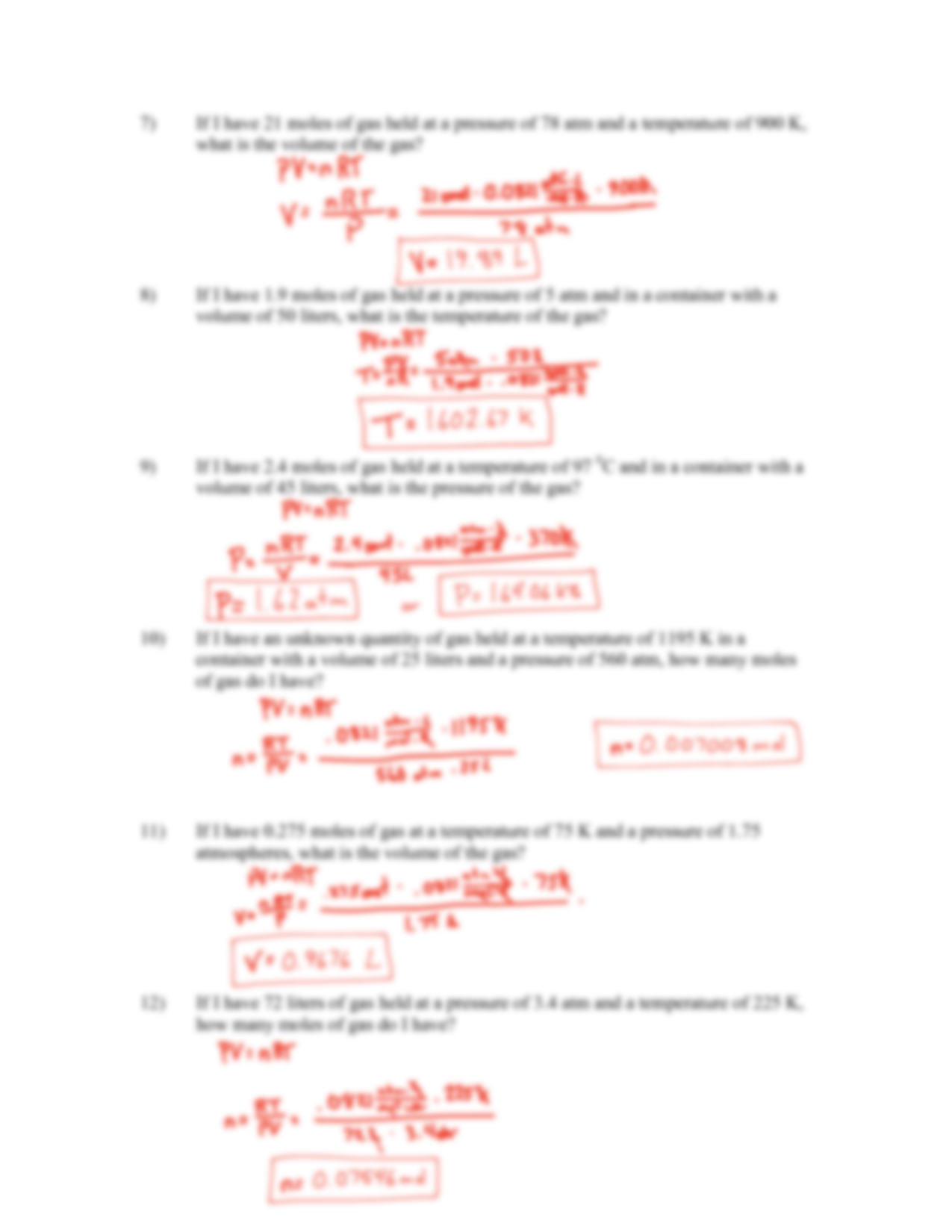
worksheets.clipart-library.com
Ohm22s Law Worksheet Answers – E-streetlight.com

www.e-streetlight.com
The Ideal Gas Law Worksheet – Worksheets For Kindergarten

worksheets.ekocraft-appleleaf.com
Worksheet # 1 – Newtons Laws Of Motion If The Statement Is True

worksheets.clipart-library.com
WORKSHEET: BOYLE'S LAW WS #1 – Pressure & Volume | Summaries Law
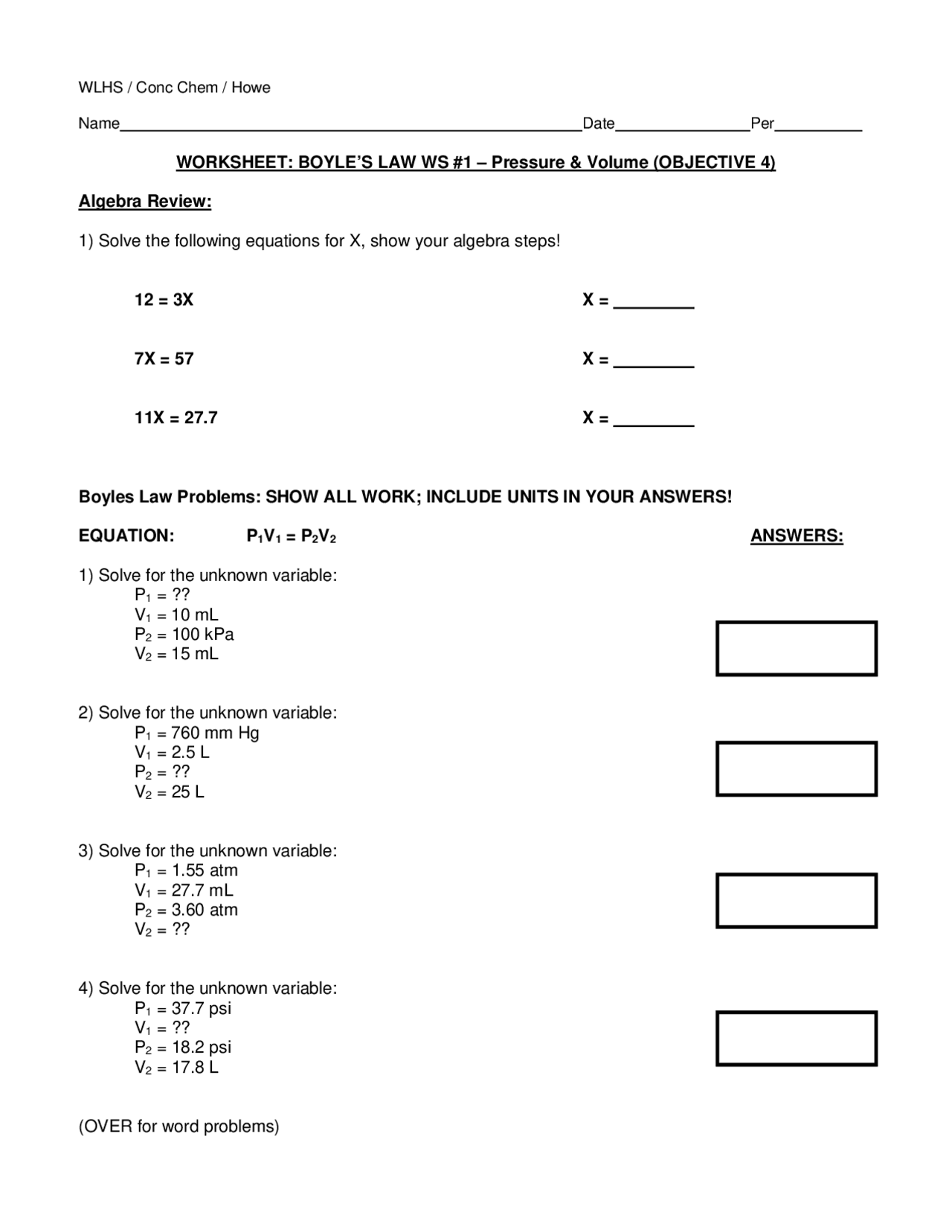
www.docsity.com
Boyles Law Worksheet – Printable Word Searches

davida.davivienda.com
9-14a,b Boyles Law And Charless Law Wkst-Key – Worksheet: Boyles
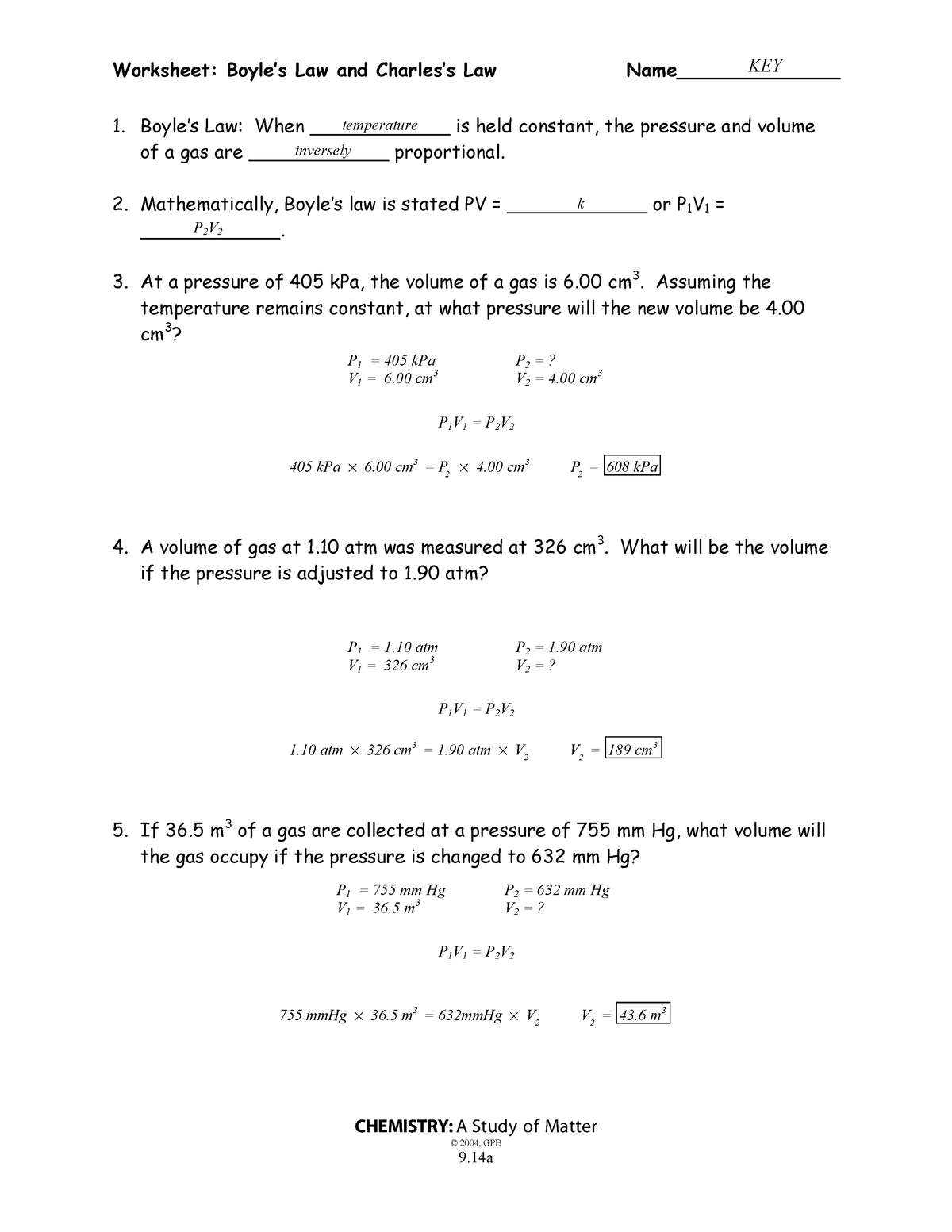
worksheets.clipart-library.com
Worksheet Boyle's Law And Charles Law – Printable Word Searches

davida.davivienda.com
SOLUTION: Ideal Gass Law Worksheet – Studypool – Worksheets Library

worksheets.clipart-library.com
boyles law and charles law worksheet. Solution: ideal gas law worksheet 2 answer. The ideal gas law worksheet