Understanding the behavior of gases, liquids, and solids can be a daunting task without a solid foundation in the principles that govern their movement and interactions. That’s where the Kinetic Molecular Theory (KMT) comes in. This powerful theory provides a simplified, yet surprisingly accurate, model for explaining the properties of matter at the molecular level. Whether you’re a high school student just beginning your exploration of chemistry or a seasoned scientist looking for a quick refresher, grasping the core concepts of KMT is crucial. That’s why mastering the topics covered in a Kinetic Molecular Theory worksheet is so important.
A well-designed Kinetic Molecular Theory worksheet will often cover several key areas. It will likely test your understanding of the assumptions of the theory, such as the idea that gas particles are in constant, random motion and that collisions between these particles are perfectly elastic. You’ll be asked to relate these assumptions to observable properties of gases, like pressure, volume, and temperature. Furthermore, the worksheet might delve into the relationship between kinetic energy and temperature, exploring how increasing temperature affects the speed and movement of particles. Finally, many KMT worksheets include questions related to the differences between ideal and real gases, highlighting the limitations of the theory under extreme conditions.
Successfully completing a Kinetic Molecular Theory worksheet requires a combination of memorization and application. You need to recall the basic tenets of the theory, but also be able to apply them to different scenarios and problems. This involves understanding how changes in temperature, pressure, or volume will affect the behavior of gas particles, and how these changes can be explained in terms of the kinetic energy and movement of the molecules. For example, a common question might ask you to explain why gases are easily compressible based on the assumptions of KMT. Another might challenge you to predict the effect of increasing temperature on the pressure of a gas in a closed container.
To help you solidify your understanding and ace those KMT-related questions, below is a sample answer key to a typical Kinetic Molecular Theory worksheet. Remember, this is just a general guide, and the specific questions on your worksheet may vary. However, the underlying principles and concepts will remain the same, so understanding these answers will give you a significant advantage.
Kinetic Molecular Theory Worksheet – Sample Answers
General Concepts
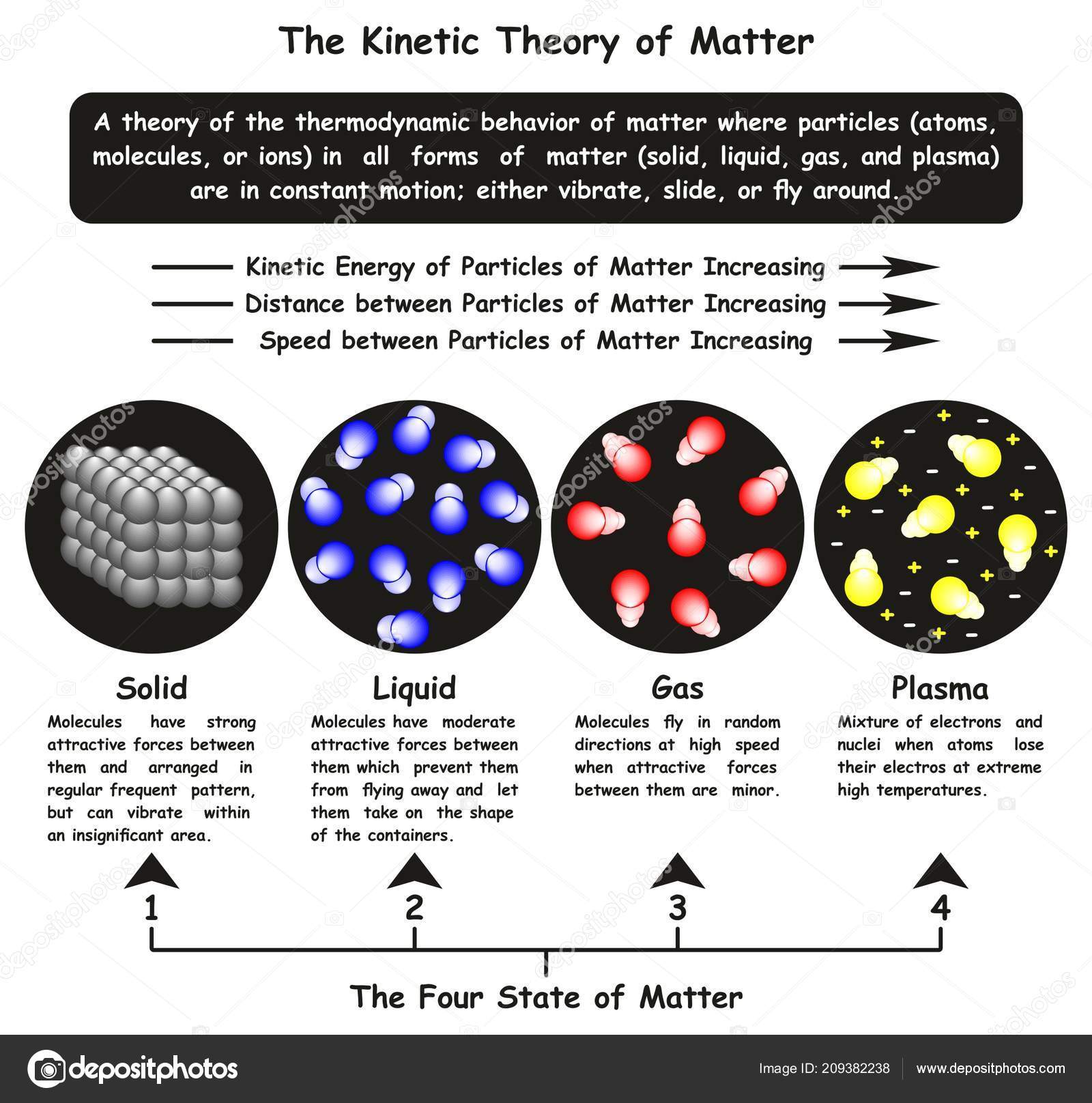
- Question 1: State the five main assumptions of the Kinetic Molecular Theory.
- Answer:
- Gases consist of a large number of tiny particles that are far apart relative to their size.
- The particles are in constant, random motion.
- Collisions between particles and with the walls of the container are perfectly elastic (no energy is lost).
- There are no forces of attraction or repulsion between the particles.
- The average kinetic energy of the particles is directly proportional to the absolute temperature (Kelvin) of the gas.
- Question 2: Explain how the Kinetic Molecular Theory explains gas pressure.
- Answer: Gas pressure is caused by the constant collisions of gas particles with the walls of the container. The more frequent and forceful these collisions, the higher the pressure.
- Question 3: What is the relationship between temperature and the average kinetic energy of gas particles?
- Answer: The average kinetic energy of gas particles is directly proportional to the absolute temperature (Kelvin). As temperature increases, the average kinetic energy of the particles increases, meaning they move faster.
- Question 4: Define diffusion and effusion. How does the Kinetic Molecular Theory explain these phenomena?
- Answer:
- Diffusion: The mixing of gases due to their random motion.
- Effusion: The escape of a gas through a small opening.
KMT explains diffusion and effusion through the constant, random motion of gas particles. Lighter particles move faster and therefore diffuse and effuse more quickly than heavier particles.
- Question 5: How does increasing the temperature of a gas affect its volume (assuming constant pressure)? Explain using KMT.
- Answer: Increasing the temperature of a gas will cause its volume to increase (assuming constant pressure). This is because the increased temperature increases the average kinetic energy of the particles, causing them to move faster and collide with the walls of the container more forcefully. To maintain constant pressure, the volume must increase, allowing the particles more space to move.
- Question 6: How does decreasing the volume of a gas affect its pressure (assuming constant temperature)? Explain using KMT.
- Answer: Decreasing the volume of a gas will cause its pressure to increase (assuming constant temperature). With less space available, the gas particles will collide with the container walls more frequently, resulting in a higher pressure.
- Question 7: Explain why gases are much more compressible than liquids or solids, based on the assumptions of KMT.
- Answer: Gases are much more compressible because their particles are far apart relative to their size (KMT assumption #1). This large amount of empty space allows the gas particles to be squeezed closer together when pressure is applied. In contrast, liquids and solids have particles that are much closer together, leaving very little empty space for compression.
Real vs. Ideal Gases
- Question 8: What are the conditions under which real gases deviate significantly from ideal behavior?
- Answer: Real gases deviate significantly from ideal behavior under high pressure and low temperature.
- Question 9: Explain why real gases deviate from ideal behavior under high pressure.
- Answer: Under high pressure, the gas particles are forced closer together. This makes the volume of the particles themselves and the intermolecular forces between them more significant, violating KMT assumptions #1 and #4.
- Question 10: Explain why real gases deviate from ideal behavior under low temperature.
- Answer: Under low temperature, the gas particles move more slowly. This allows intermolecular forces of attraction to become more significant, pulling the particles closer together and deviating from the assumption of no intermolecular forces (KMT assumption #4).
By carefully reviewing these sample answers and understanding the underlying reasoning, you’ll be well-prepared to tackle any Kinetic Molecular Theory worksheet that comes your way. Good luck with your studies!
If you are searching about Kinetic Molecular Theory Worksheet – Printable PDF Template you’ve visit to the right page. We have 20 Pics about Kinetic Molecular Theory Worksheet – Printable PDF Template like Kinetic Molecular Theory Worksheet – Printable PDF Template, 50 Kinetic Molecular theory Worksheet | Chessmuseum Template Library and also MOLECULAR GEOMETRY AND Polarity ANSWER KEY | Exams Chemistry | Docsity. Here you go:
Kinetic Molecular Theory Worksheet – Printable PDF Template

martinlindelof.com
SOLUTION: Chemistry 112 Kinetic Molecular Theory – Studypool

www.studypool.com
Kinetic And Potential Energy Worksheet Answer Keyk O – Kinetic And

worksheets.clipart-library.com
Lab 3b Pdf PHET Gases Kinetic Molecular Theory – Phet – KINETIC
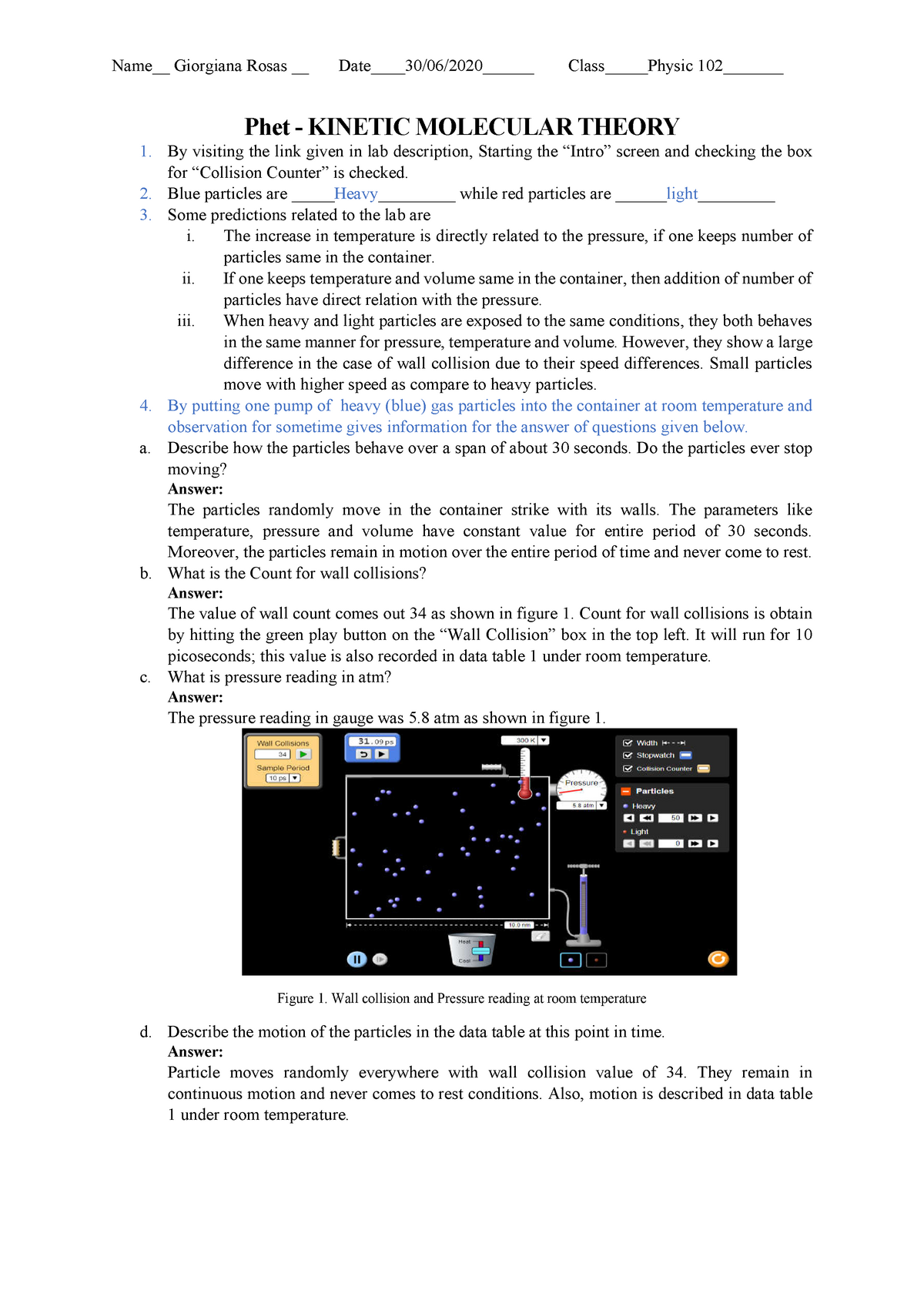
worksheets.clipart-library.com
SOLUTION: 5 The Kinetic Molecular Theory – Studypool

www.studypool.com
Kinetic Energy Practice Worksheet | Study Notes Physics | Docsity

worksheets.clipart-library.com
The Kinetic Molecular Theory Chemistry I – Vrogue.co
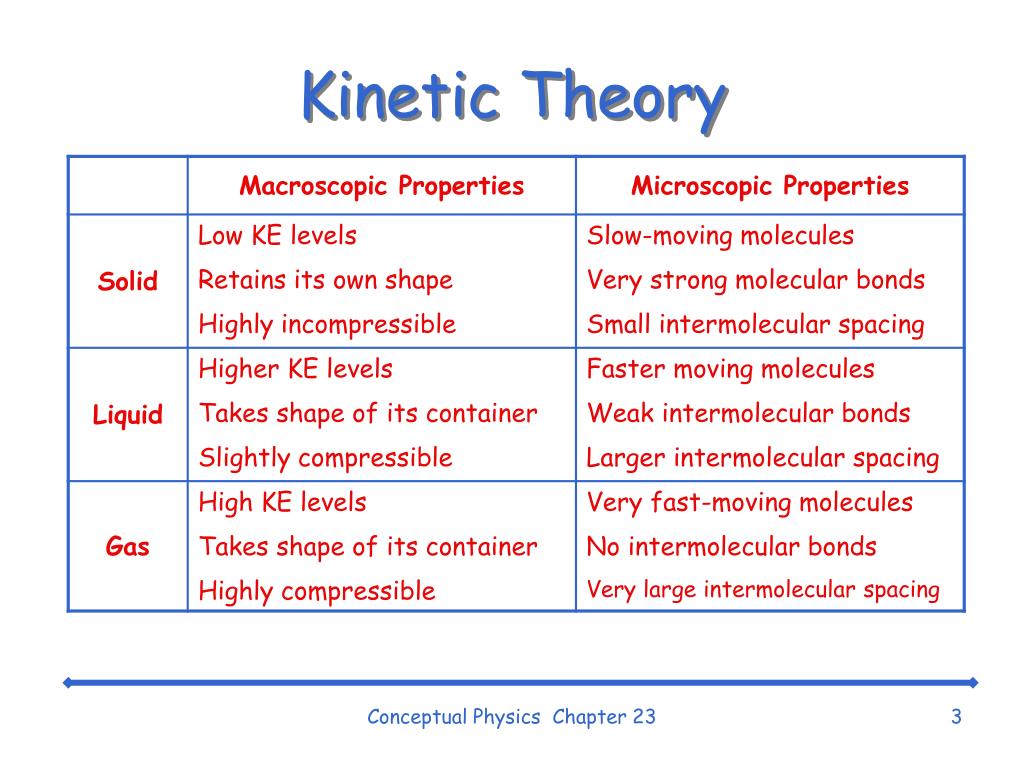
www.vrogue.co
POGIL: Kinetic Molecular Theory | Study Notes Chemistry | Docsity
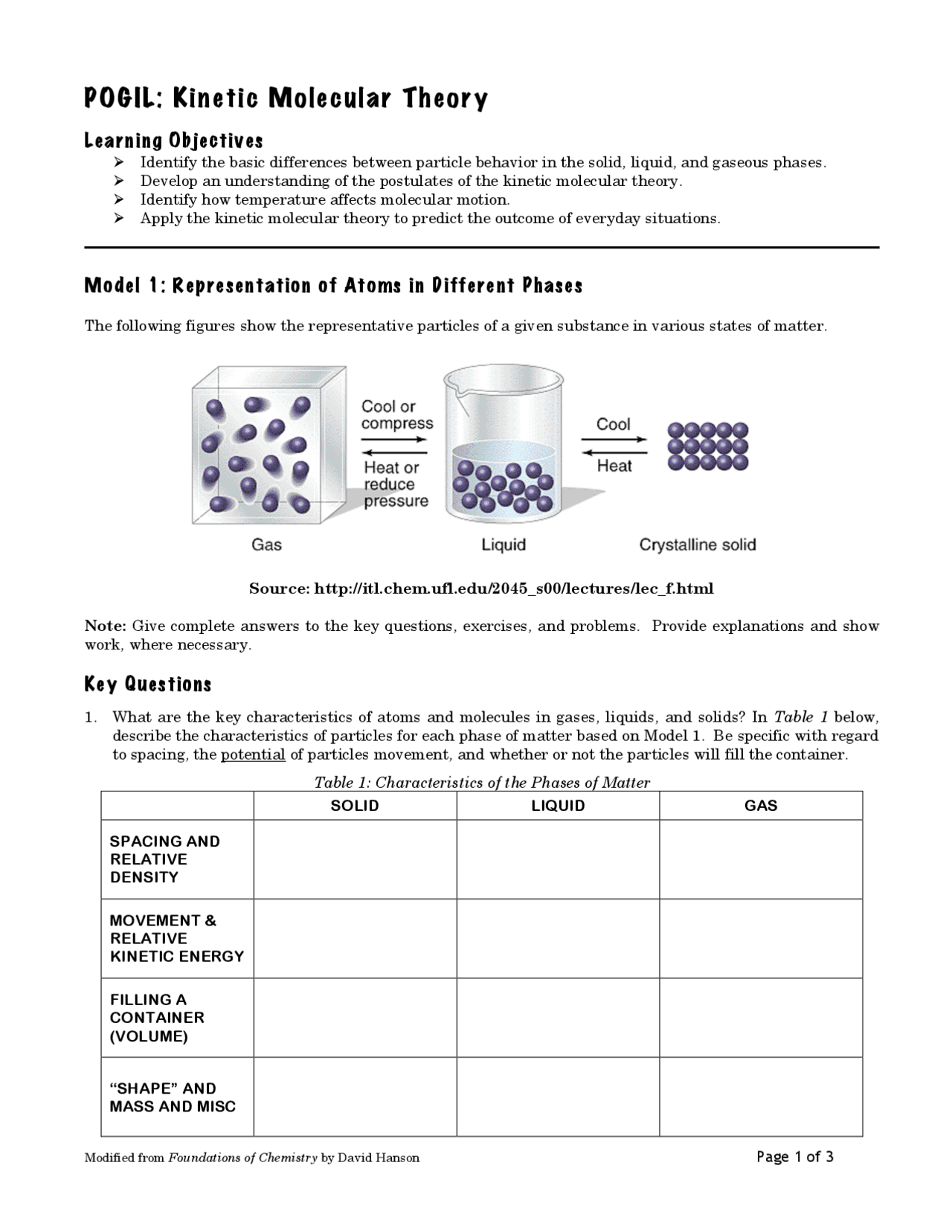
worksheets.clipart-library.com
Kinetic Molecular Theory Of Gases – Worksheets Library
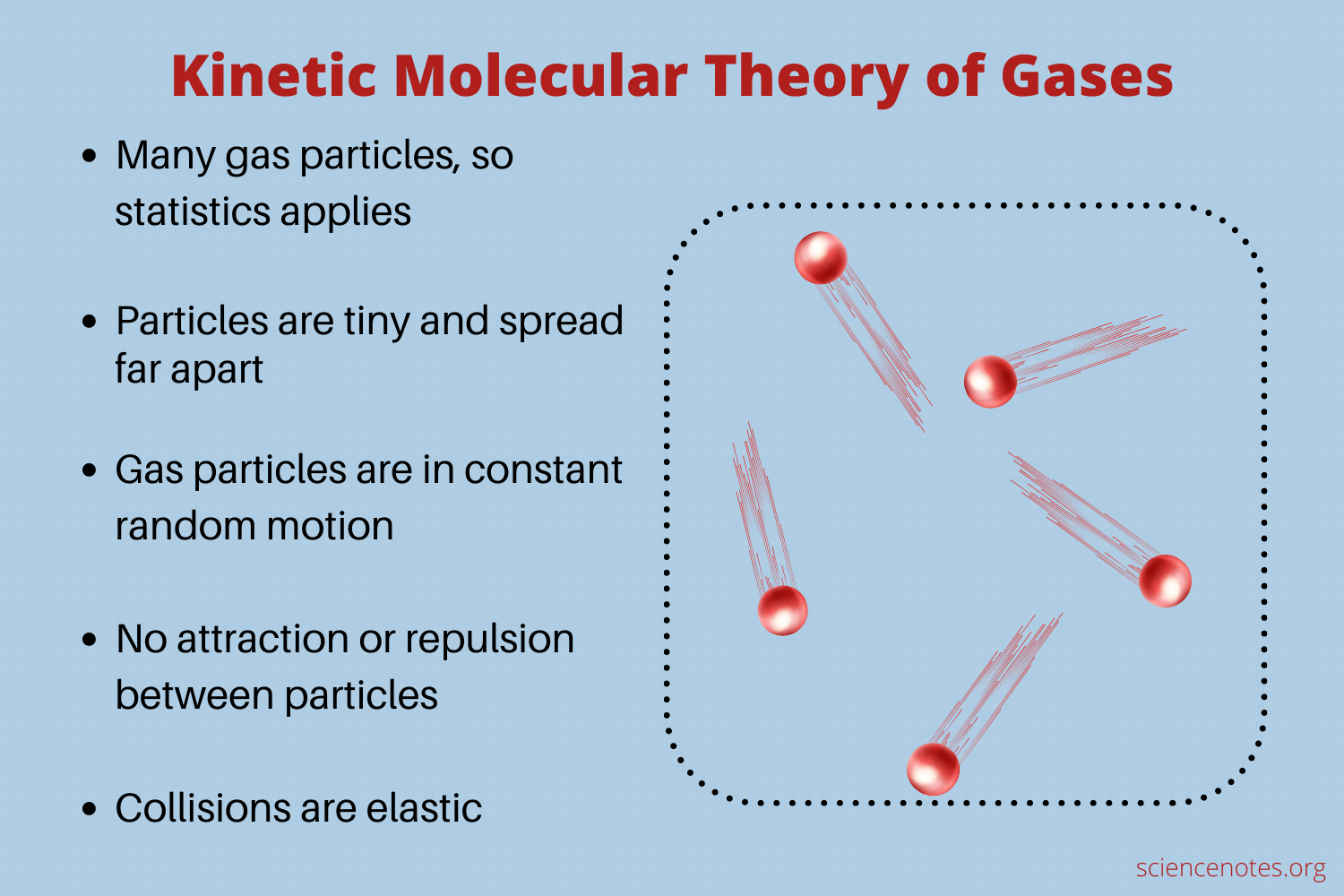
worksheets.clipart-library.com
Kinetic Molecular Theory Worksheet – Owhentheyanks.com

www.owhentheyanks.com
What Is Kinetic Molecular Theory Of Gas – Free Worksheets Printable
lisaworksheets.com
Quiz & Worksheet – The Kinetic Theory Of Matter | Study.com

worksheets.clipart-library.com
What Is Kinetic Molecular Theory Kmt – Free Worksheets Printable
.jpg)
lisaworksheets.com
What Is Kinetic Molecular Theory Kmt – Free Worksheets Printable

lisaworksheets.com
50 Kinetic Molecular Theory Worksheet | Chessmuseum Template Library

www.pinterest.com
Quiz & Worksheet – Properties Of Gases With The Kinetic Molecular
worksheets.clipart-library.com
MOLECULAR GEOMETRY AND Polarity ANSWER KEY | Exams Chemistry | Docsity

worksheets.clipart-library.com
KINETIC AND POTENTIAL ENERGY WORKSHEET | Exercises Physics | Docsity

worksheets.clipart-library.com
Kinetic Molecular Theory Worksheet – ChemTribe

chemtribe.com
Kinetic Theory Worksheet – Worksheets For Kindergarten

worksheets.ekocraft-appleleaf.com
molecular geometry and polarity answer key. Pogil: kinetic molecular theory. Kinetic molecular theory worksheet – owhentheyanks.com