Welcome to a comprehensive resource for understanding atomic structure! Mastering atomic structure is fundamental to grasping chemistry and other scientific disciplines. From understanding the basic building blocks of matter to predicting chemical behavior, a solid grasp of atomic structure is essential. Many students encounter challenges when first learning about protons, neutrons, electrons, atomic number, mass number, isotopes, ions, and electron configuration. A well-designed worksheet can be invaluable in reinforcing these concepts. But simply completing the worksheet isn’t enough; understanding the answers is crucial. This post provides an “Atomic Structure Worksheet Answer Key” to help you solidify your understanding of this vital topic. Whether you are a student preparing for an exam or a teacher looking for a helpful resource, this guide offers a detailed explanation of atomic structure worksheet answers. We’ll delve into the logic behind each answer, making it easier to learn and remember the key principles.
Understanding Atomic Structure: A Detailed Answer Key
This section offers the answer key to a typical atomic structure worksheet. Remember, understanding why an answer is correct is just as important as knowing the answer itself. Therefore, alongside each answer, we provide a brief explanation to enhance your learning process.
The following answers are based on a worksheet that covers basic concepts such as identifying atomic number, mass number, number of protons, neutrons, and electrons in various elements and ions. It also touches upon concepts such as isotopes, and basic electron configuration.
Answer Key:
- Question 1: Determine the number of protons, neutrons, and electrons in an atom of Oxygen-16.
- Answer:
- Protons: 8
- Neutrons: 8
- Electrons: 8
Explanation: Oxygen has an atomic number of 8, which means it has 8 protons. Oxygen-16 has a mass number of 16. The number of neutrons is calculated as mass number minus atomic number (16 – 8 = 8). In a neutral atom, the number of electrons equals the number of protons.
- Answer:
- Protons: 12
- Neutrons: 12
- Electrons: 10
Explanation: Magnesium (Mg) has an atomic number of 12, meaning it has 12 protons. A typical isotope of Magnesium (Magnesium-24) has a mass number of 24. Thus it has (24-12 = 12) neutrons. The “+2” charge indicates that the magnesium atom has lost two electrons. Thus the number of electrons is 12 – 2 = 10.
- Answer: 1s22s22p63s23p64s1
- Explanation: Potassium has 19 electrons. The electron configuration follows the Aufbau principle, filling the lowest energy levels first. The sequence is 1s, 2s, 2p, 3s, 3p, 4s. s orbitals can hold a maximum of 2 electrons, and p orbitals can hold a maximum of 6 electrons.
- Answer: Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. Example: Carbon-12 and Carbon-14 are isotopes of carbon. Both have 6 protons, but Carbon-12 has 6 neutrons, while Carbon-14 has 8 neutrons.
- Explanation: The key to understanding isotopes is recognizing that the number of protons defines the element. Changing the number of neutrons only changes the mass of the atom, not its identity.
- Answer: Chlorine-35 (Cl-35)
- Explanation: The number of protons determines the element. An element with 17 protons is chlorine (Cl). The mass number is the sum of protons and neutrons (17 + 18 = 35). Therefore, the element is Chlorine-35.
- Answer: The atomic number represents the number of protons in the nucleus of an atom, which defines the element. The mass number represents the total number of protons and neutrons in the nucleus of an atom.
- Explanation: Think of the atomic number as the “identity card” of an element; it never changes for a given element. The mass number, on the other hand, can vary due to the presence of different isotopes.
- Answer: The Bohr model should show a nucleus with 7 protons and 7 neutrons. There should be two electron shells. The first shell should contain 2 electrons, and the second shell should contain 5 electrons.
- Explanation: Nitrogen has an atomic number of 7, meaning it has 7 protons and in a neutral atom, 7 electrons. Its mass number is typically 14, meaning it has (14-7 = 7) neutrons. The first electron shell can hold a maximum of 2 electrons, and the second can hold a maximum of 8. Therefore, nitrogen will have 2 electrons in the first shell and 5 in the second shell.
- Answer: Valence electrons are the electrons located in the outermost electron shell of an atom. They are important because they determine the chemical properties of an atom and how it will interact with other atoms to form chemical bonds.
- Explanation: Valence electrons are “active participants” in chemical reactions. Elements with similar numbers of valence electrons tend to have similar chemical behaviors.
By working through these questions and understanding the reasoning behind the answers, you can build a strong foundation in atomic structure. Remember to consult your textbook, teacher, or online resources if you need further clarification. Keep practicing, and you’ll master atomic structure in no time!
If you are searching about 12 Label An Atom Worksheet – Free PDF at worksheeto.com you’ve came to the right web. We have 20 Images about 12 Label An Atom Worksheet – Free PDF at worksheeto.com like Atomic Structure Review sheet with Answers | Exercises Chemistry, Free Printable Atomic Structure Worksheets – Worksheets Library and also Atomic Structure Review sheet with Answers | Exercises Chemistry. Here you go:
12 Label An Atom Worksheet – Free PDF At Worksheeto.com

www.worksheeto.com
Free Printable Atomic Structure Worksheets – Worksheets Library

worksheets.clipart-library.com
Atomic Structure Worksheet Answer Key – E-streetlight.com
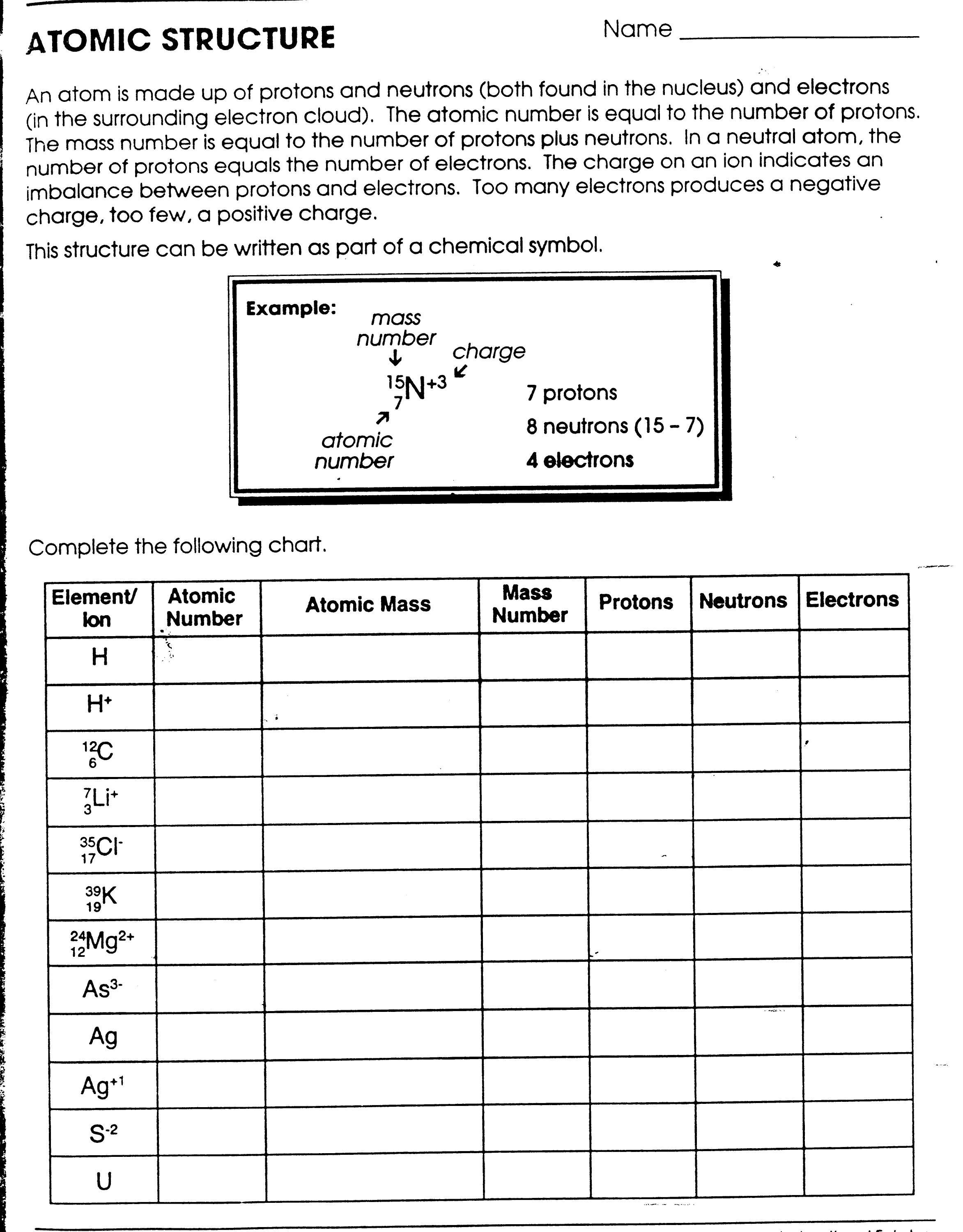
www.e-streetlight.com
Atomic Structure Atoms Inside Out Worksheet Answers – Printable Word

davida.davivienda.com
Structure Of An Atom Worksheet

dbdalrymplelockable.z21.web.core.windows.net
Atomic Structure Worksheet Chemistry

lessonschoolcheckmates.z14.web.core.windows.net
Drawing Atoms Worksheet Answer Key
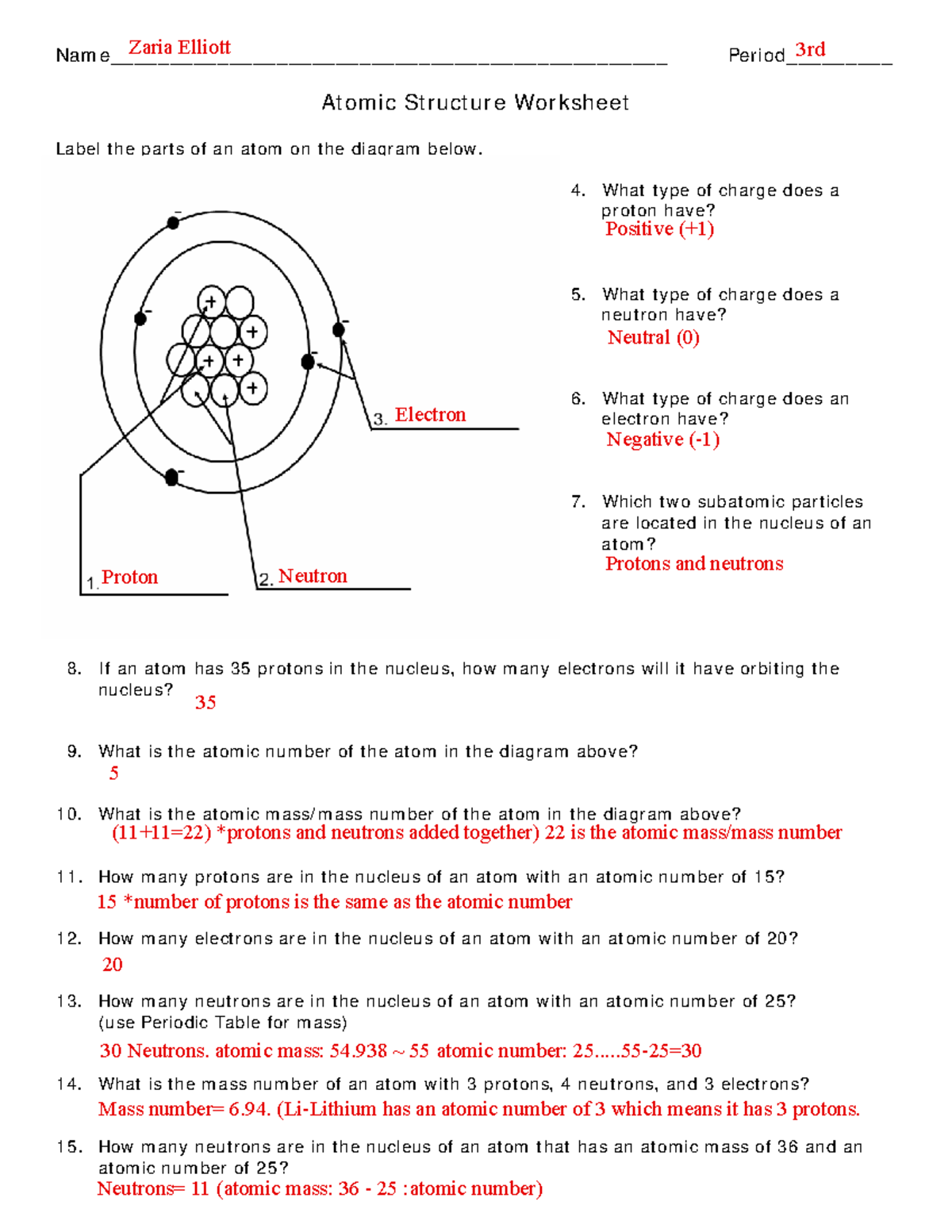
www.owhentheyanks.com
Atomic Structure Worksheet Answers – E-streetlight.com
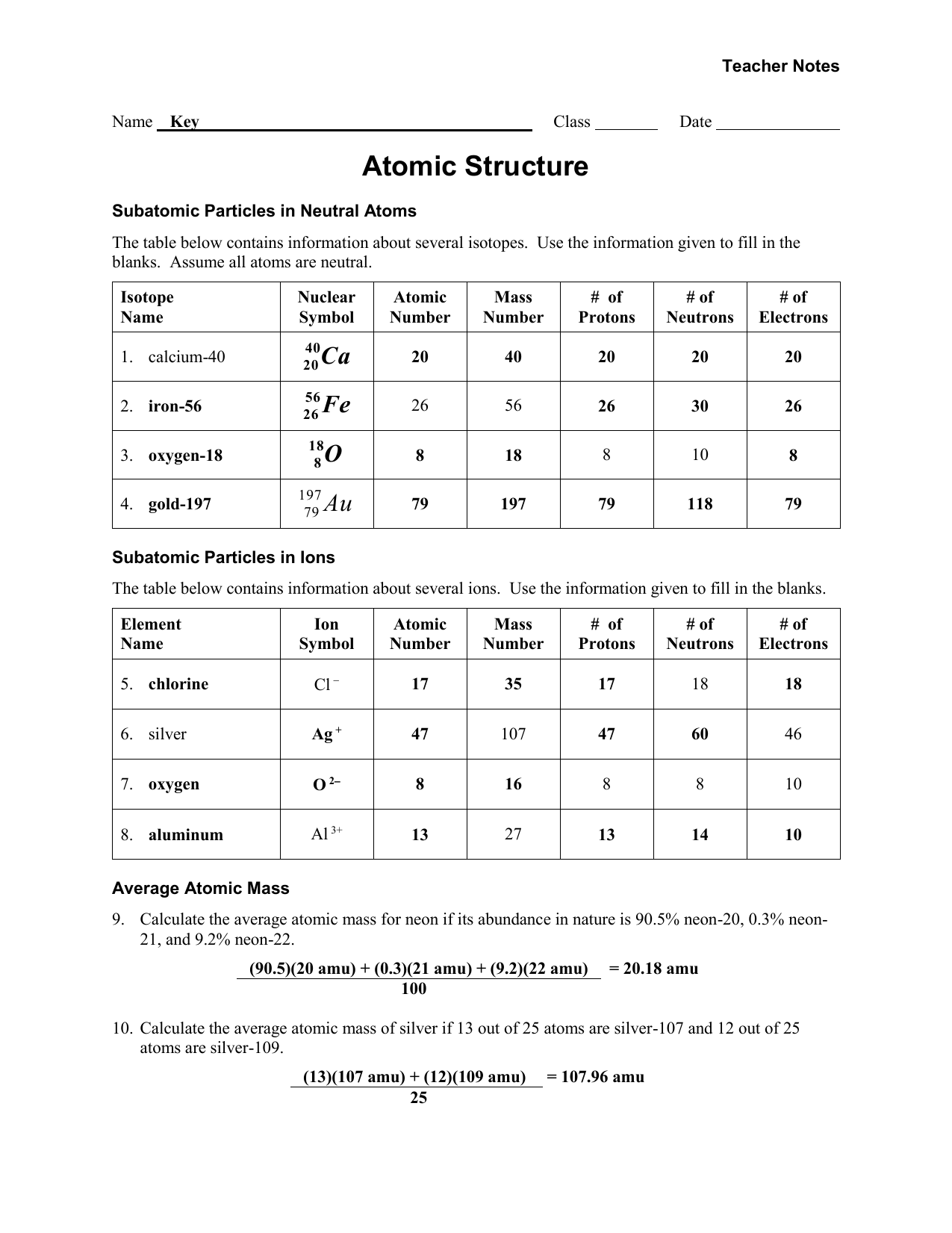
www.e-streetlight.com
Atomic Structure Practice Worksheet Answers – E-streetlight.com
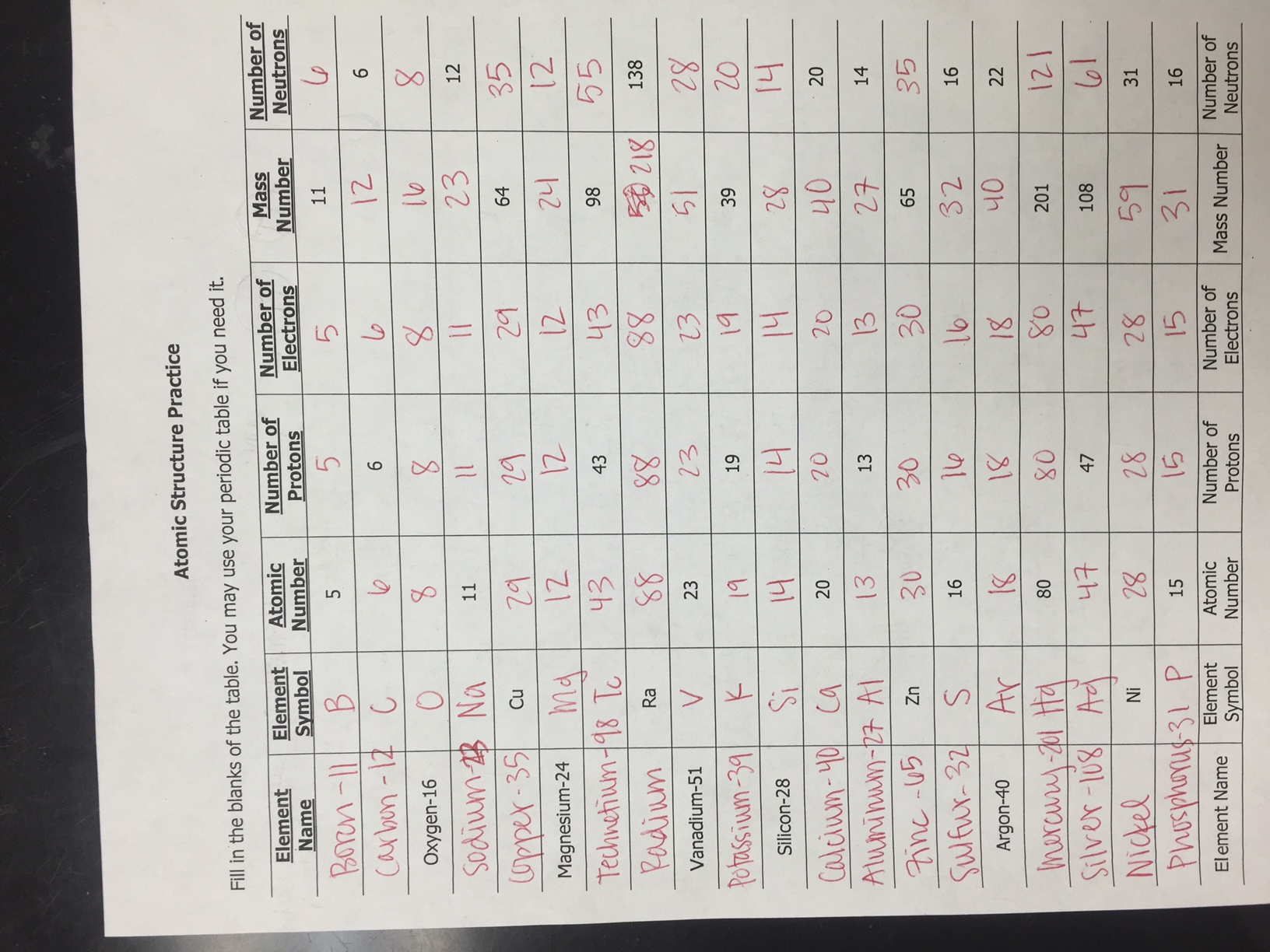
www.e-streetlight.com
Atomic Structure Worksheet Answer Key – Proworksheet.my.id
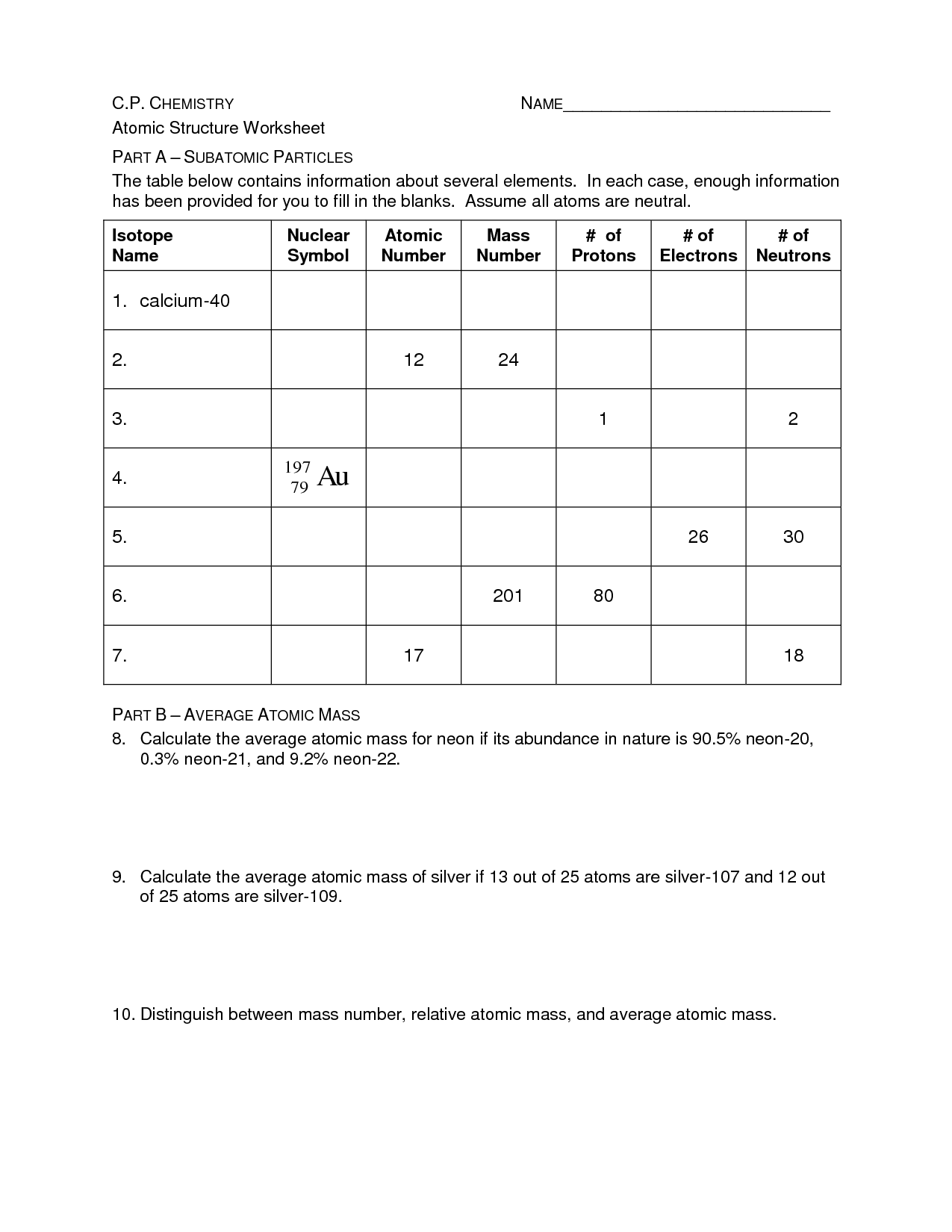
www.proworksheet.my.id
Atomic Structure Worksheet #3 – Studocu

www.studocu.com
History Of The Atomic Model Worksheet History Of Atomic Theo

eintauschxpxlessonmedia.z14.web.core.windows.net
Sort Elements Chart With Electrons PDF Storyboard
www.storyboardthat.com
Atomic Structure Worksheet Answer Key – Proworksheet.my.id
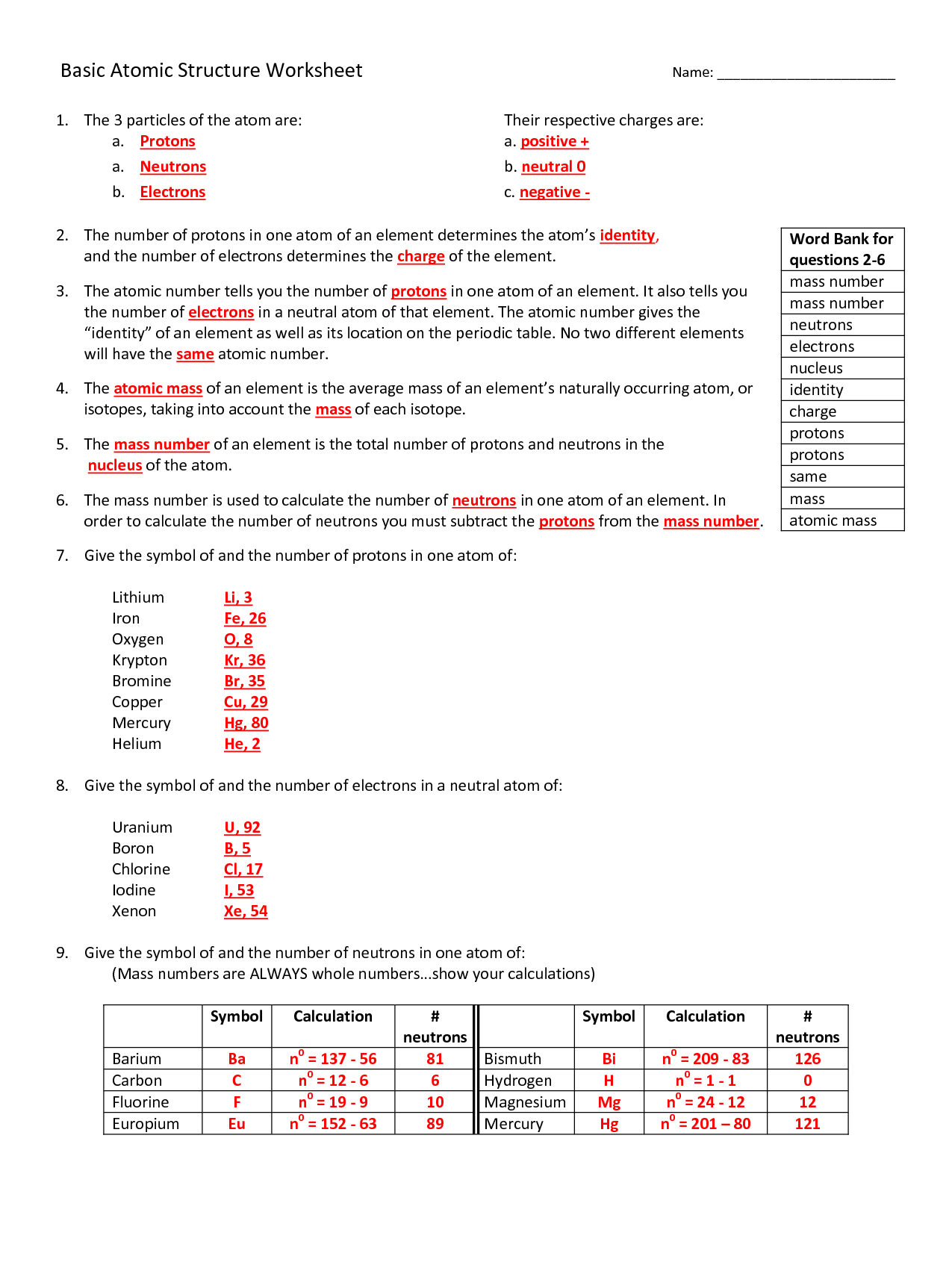
www.proworksheet.my.id
Atomic Structure Review Sheet With Answers | Exercises Chemistry

worksheets.clipart-library.com
Atomic Structure Worksheet Pdf – Pro Worksheet

www.proworksheet.my.id
SOLUTION: Parrish Community High School Chemistry Atomic Structure

worksheets.clipart-library.com
Atomic Structure Review Sheet With Answers | Exercises Chemistry

worksheets.clipart-library.com
Basic Atomic Structure Worksheet

learningschoolportulacq4.z22.web.core.windows.net
Atomic Structure Wkst – Science – Worksheet: Atomic Structure
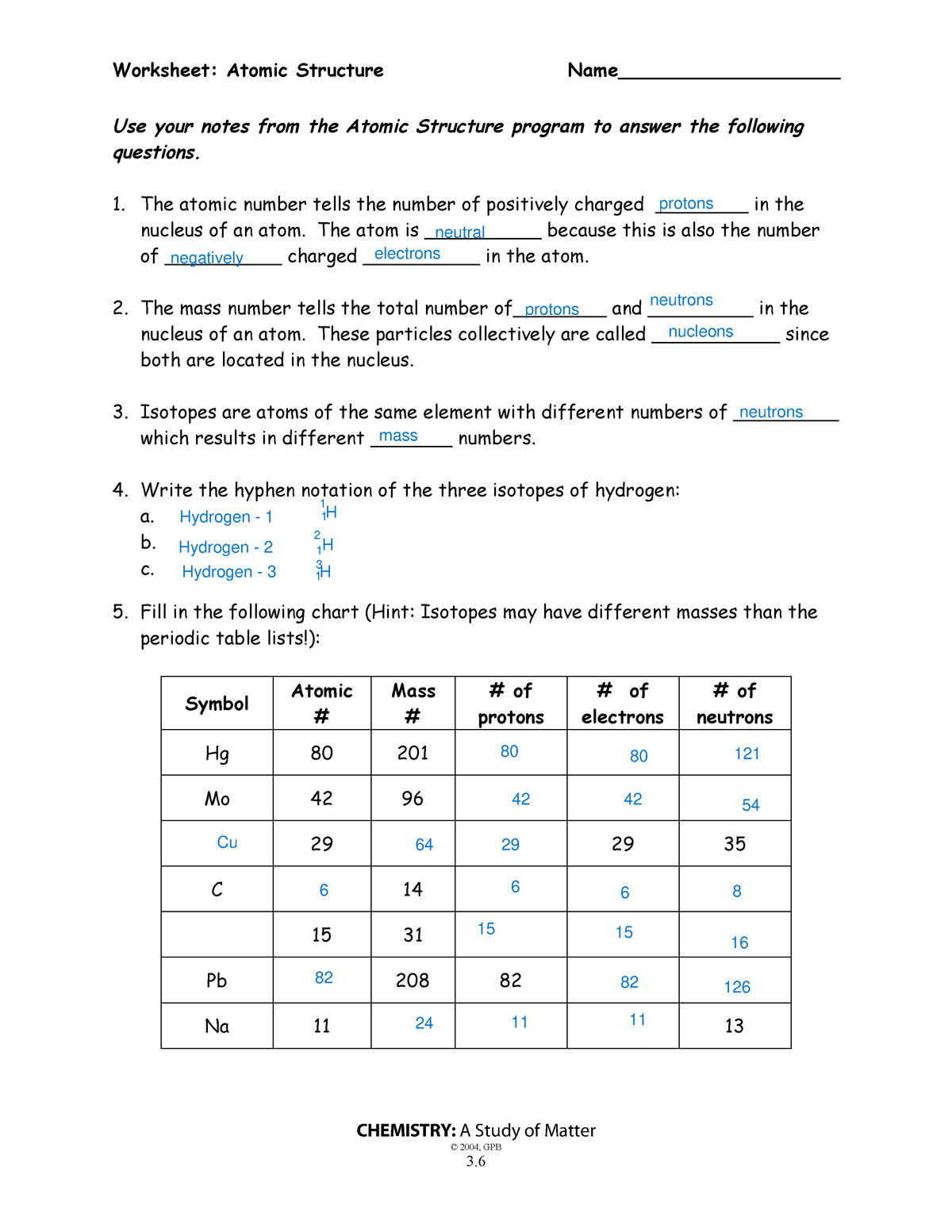
worksheets.clipart-library.com
Atomic structure review sheet with answers. Free printable atomic structure worksheets. Atomic structure worksheet answer key