Struggling to visualize chemical bonds? One of the best ways to understand how atoms share and transfer electrons is through Lewis Dot Structures! This method, also known as electron dot diagrams, provides a simple and effective visual representation of valence electrons and how they participate in bonding. Completing a Lewis Dot Structure worksheet can be a fantastic way to master this crucial chemistry concept. This post will explore the benefits of using these worksheets and provide you with example answers to help you ace your next chemistry quiz!
Why Use a Lewis Dot Structure Worksheet?
Lewis Dot Structure worksheets are invaluable tools for learning and reinforcing your understanding of chemical bonding. Here’s why:
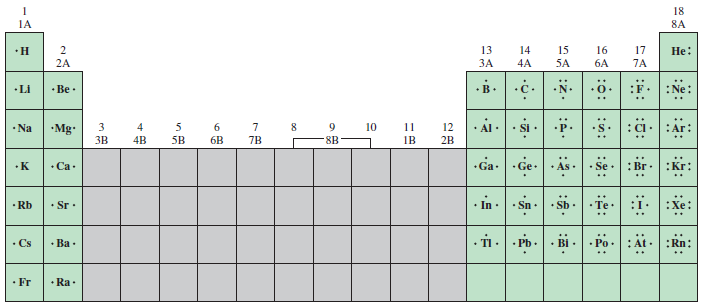
* **Visualization of Valence Electrons:** They clearly show the number of valence electrons (electrons in the outermost shell) surrounding an atom, which are the key players in chemical bonding. Understanding this concept is fundamental to predicting how atoms will interact.
* **Predicting Molecular Geometry:** While Lewis Dot Structures don’t perfectly represent 3D geometry, they provide a foundational understanding that helps you predict the arrangement of atoms in a molecule using theories like VSEPR (Valence Shell Electron Pair Repulsion). Seeing where the lone pairs of electrons are located is critical for determining overall molecular shape.
* **Understanding Bonding Patterns:** By drawing Lewis Dot Structures, you can visualize how atoms achieve stable octets (or duets for hydrogen). This helps you understand the formation of single, double, and triple bonds.
* **Identifying Lone Pairs:** Lone pairs of electrons play a significant role in the reactivity and properties of molecules. Lewis Dot Structures clearly show these non-bonding electron pairs.
* **Problem-Solving Practice:** Worksheets provide structured practice, allowing you to apply the rules for drawing Lewis Dot Structures and refine your problem-solving skills. They provide a guided learning approach.
* **Building a Foundation for Advanced Concepts:** Lewis Dot Structures serve as a foundation for understanding more complex concepts such as resonance structures, formal charge, and molecular orbital theory.
Example Lewis Dot Structure Solutions
Below are example solutions for common molecules and ions. Remember, the steps to draw a Lewis Dot Structure include: 1) Calculate the total number of valence electrons. 2) Draw the skeletal structure with the least electronegative element in the center (usually). 3) Distribute electrons to surrounding atoms to fulfill the octet rule (or duet rule for hydrogen). 4) Place any remaining electrons on the central atom. 5) If the central atom does not have an octet, form multiple bonds.
Examples:
-
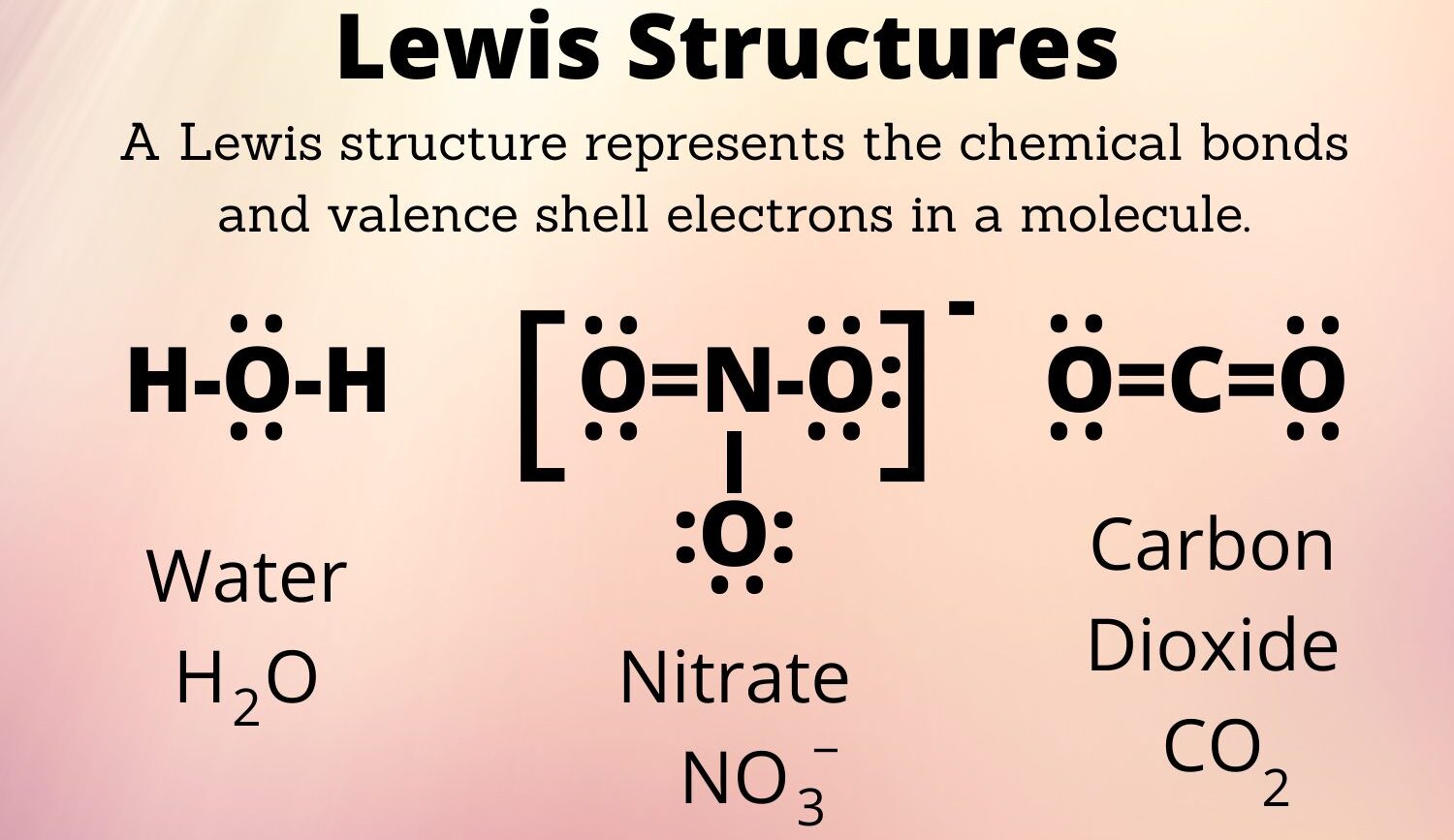
1. Water (H2O):
- Total valence electrons: (2 x 1) + 6 = 8
- Central atom: Oxygen (O)
- Lewis Dot Structure:

-
2. Carbon Dioxide (CO2):
- Total valence electrons: 4 + (2 x 6) = 16
- Central atom: Carbon (C)
- Lewis Dot Structure:

-
3. Ammonia (NH3):
- Total valence electrons: 5 + (3 x 1) = 8
- Central atom: Nitrogen (N)
- Lewis Dot Structure:

-
4. Methane (CH4):
- Total valence electrons: 4 + (4 x 1) = 8
- Central atom: Carbon (C)
- Lewis Dot Structure:

-
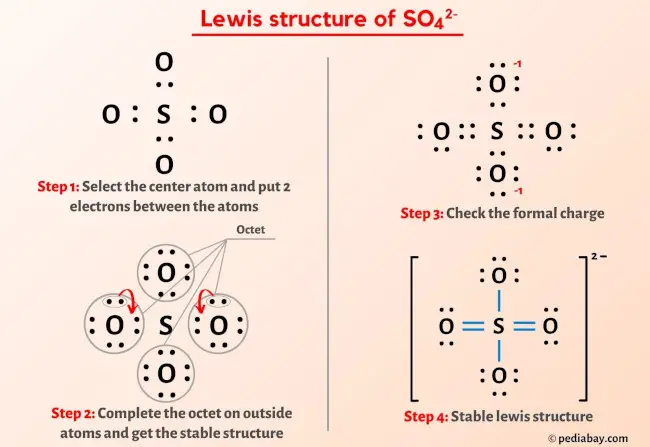
5. Sulfate Ion (SO42-):
- Total valence electrons: 6 + (4 x 6) + 2 = 32
- Central atom: Sulfur (S)
- Lewis Dot Structure:

Remember that these are just a few examples. A Lewis Dot Structure worksheet will typically contain a wider variety of molecules and ions to challenge your understanding and provide ample practice. When working on these worksheets, always double-check your work to ensure you have the correct number of valence electrons and that each atom (except for hydrogen) has a complete octet.
Mastering Lewis Dot Structures is a cornerstone of understanding chemical bonding. Embrace the challenge, use worksheets for practice, and you’ll be well on your way to succeeding in chemistry!
If you are searching about Lewis Structure of PCl5? [with free study guide and video] you’ve came to the right web. We have 20 Pictures about Lewis Structure of PCl5? [with free study guide and video] like Structure de Lewis : définition et explications – AquaPortail, Lewis Dot Structure: Definition, Examples, and Drawing and also THS-Quimica: Estructuras de lewis de los elementos | UTN-FRRQ. Here you go:
Lewis Structure Of PCl5? [with Free Study Guide And Video]
![Lewis Structure of PCl5? [with free study guide and video]](https://www.aceorganicchem.com/blog/wp-content/uploads/2023/05/PCl5-Lewis-puzzle-.jpg)
www.aceorganicchem.com
Structure De Lewis : Définition Et Explications – AquaPortail

www.aquaportail.com
Estructuras De Lewis De óxidos, Oxácidos Y Sus Aniones – TRIPLENLACE

triplenlace.com
HOCl Lewis Structure: How To Draw Lewis Dot Structure – YouTube

www.youtube.com
25 Unbelievable Facts About Ramsey Lewis – Facts.net
facts.net
Lewis About Life

ar.inspiredpencil.com
THS-Quimica: Estructuras De Lewis De Los Elementos | UTN-FRRQ
frrq.cvg.utn.edu.ar
The Lewis Structures Of C2H4O [with Free Study Guide And Video]
![The Lewis Structures of C2H4O [with free study guide and video]](https://www.aceorganicchem.com/blog/wp-content/uploads/2023/05/acetaldeyde-step-method.jpg)
biochemhelp.com
SO4 2- Lewis Structure In 5 Steps (With Images)
pediabay.com
Portrait Of G. N. Lewis, Approx. 1930. – Pictures And Illustrations

scarc.library.oregonstate.edu
F2O Lewis Structure: How To Draw The Lewis Structure For F2O

www.youtube.com
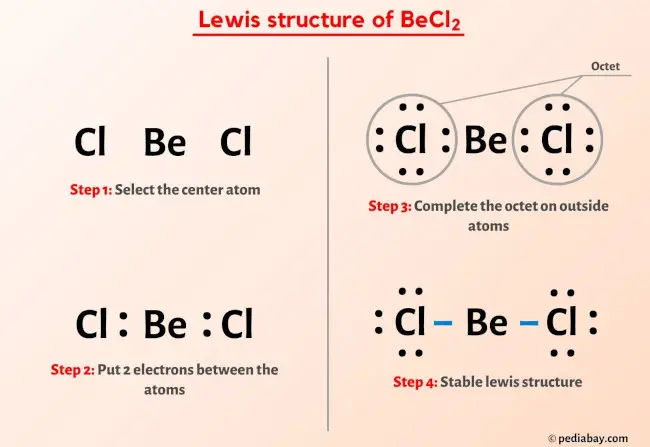
BeCl2 Lewis Structure In 6 Steps (With Images)
pediabay.com
Hướng Dẫn Vẽ So2 Lewis Structure đơn Giản Và Chi Tiết Nhất
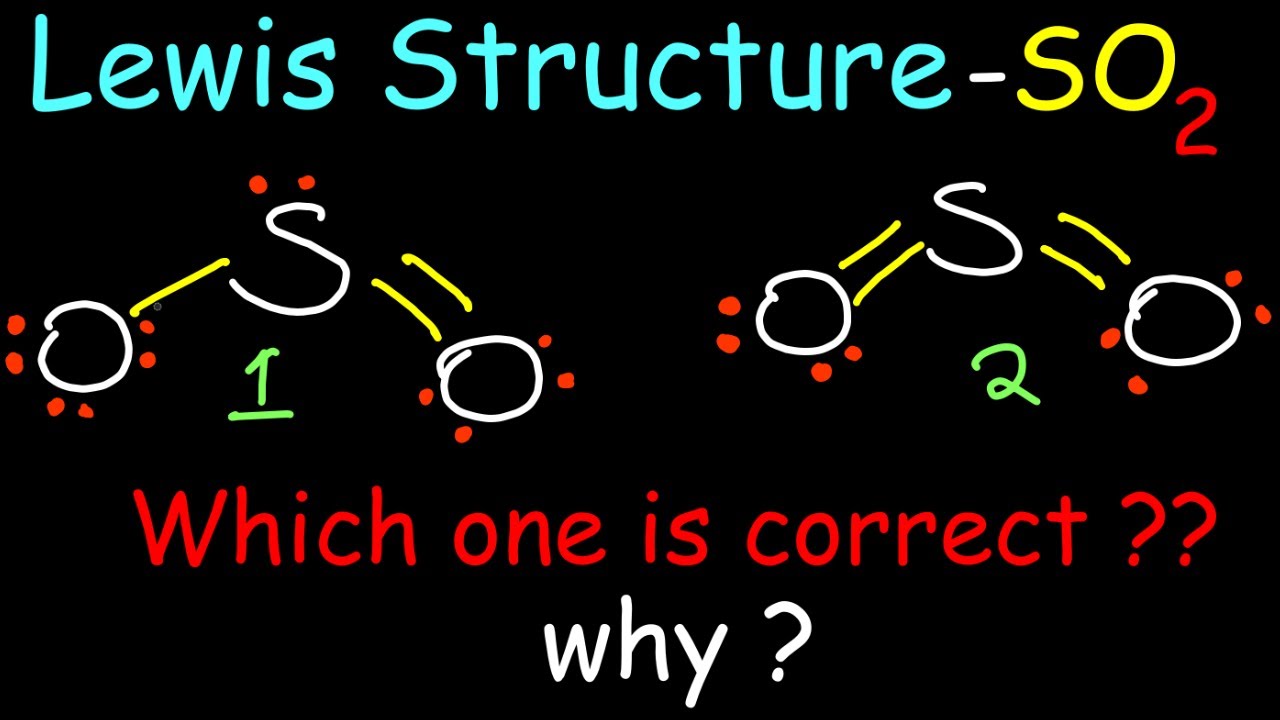
xaydungso.vn
¿Cuáles Son Las Aportaciones Del Trabajo De Lewis? – Nueva Escuela
nuevaescuelamexicana.sep.gob.mx
David Coulthard's Theory On Lewis Hamilton's Mercedes Contract Delay

www.planetf1.com
Lewis Structure Of H2CO [with Video And Free Study Guide]
![Lewis Structure of H2CO [with video and free study guide]](https://www.aceorganicchem.com/blog/wp-content/uploads/2023/05/H2Co-puzzle-peices.jpg)
aceorganicchem.com
In The Space Below Draw A Reasonable Lewis Electron D – Vrogue.co
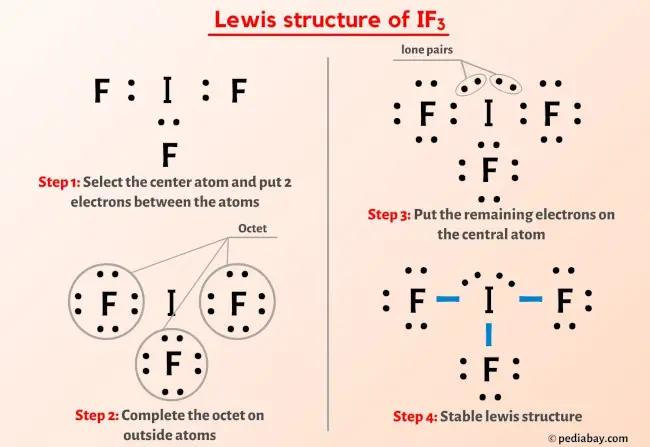
www.vrogue.co
Lewis Symbols And Structures | Chemistry I | | Course Hero
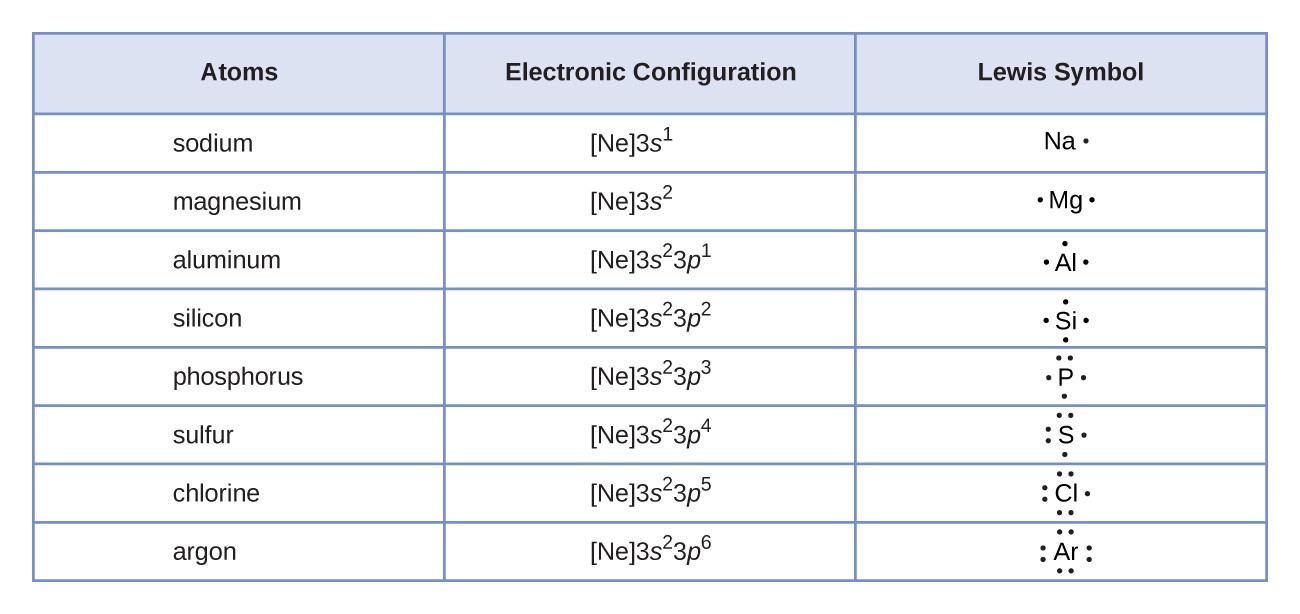
www.coursehero.com
Lewis Dot Structure: Definition, Examples, And Drawing

www.chemistrylearner.com
AsF5 Lewis Structure, Geometry, And Hybridization – Chemistry Steps
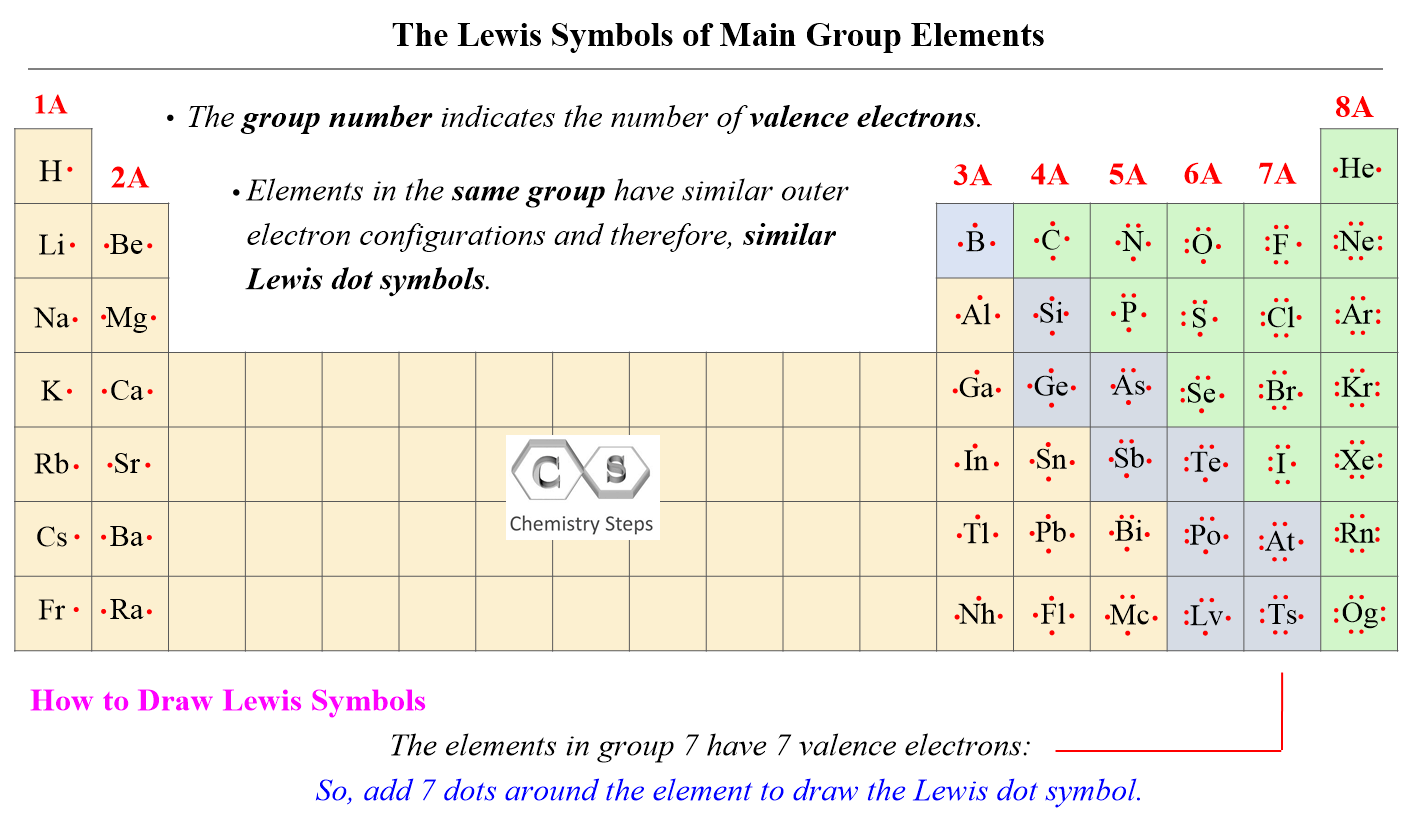
general.chemistrysteps.com
F2o lewis structure: how to draw the lewis structure for f2o …. lewis structure of pcl5? [with free study guide and video]. Lewis dot structure: definition, examples, and drawing