Alright, students! Time to brush up on your understanding of the building blocks of everything: atoms! We’re diving deep into the fascinating world of atomic structure with a comprehensive review worksheet designed to solidify your knowledge of protons, neutrons, electrons, atomic number, mass number, isotopes, ions, and more. This worksheet is crucial for laying a strong foundation for more advanced chemistry topics, so let’s make sure you’ve got a firm grasp on these fundamentals. Don’t just memorize the answers; strive to understand the underlying principles behind each concept. Think about why the atomic number defines an element, or how the number of neutrons affects an atom’s mass. This understanding will serve you well in future chemistry studies.
Remember, the atom isn’t just a tiny, indivisible sphere. It’s a complex system with a positively charged nucleus at its center, containing protons and neutrons. Orbiting this nucleus are negatively charged electrons, residing in specific energy levels or shells. These components and their interactions dictate the chemical properties of elements and how they bond to form molecules. Knowing how to determine the number of protons, neutrons, and electrons from the atomic number and mass number is essential for predicting an element’s behavior.
This worksheet will help you practice: identifying the number of subatomic particles in an atom or ion, calculating the average atomic mass of an element given the abundance of its isotopes, understanding the relationship between the number of valence electrons and an element’s chemical reactivity, and differentiating between isotopes and ions. Make sure you understand how electron configurations are determined and what information they provide. Pay special attention to exceptions to the Aufbau principle and Hund’s rule. Practicing these types of questions will provide you with a strong understanding of atomic structure and its applications.
Take your time, review your notes, and consult your textbook if needed. Understanding atomic structure is paramount to understanding all of chemistry, so put in the effort to master these core concepts now. Good luck!
Atomic Structure Review Worksheet Answers
Below are the answers to a typical Atomic Structure Review Worksheet. Your worksheet may have slightly different questions, but the concepts tested will be similar. Remember to understand the reasoning behind each answer, not just memorize the solution!
Key Concepts Covered:
- Identifying subatomic particles (protons, neutrons, electrons)
- Determining atomic number and mass number
- Understanding isotopes and ions
- Calculating average atomic mass
- Writing electron configurations
Answers:
-
Question: An atom has 17 protons, 18 neutrons, and 17 electrons. What is the element, its mass number, and its charge?
Answer:- Element: Chlorine (Cl)
- Mass Number: 35 (17 protons + 18 neutrons)
- Charge: 0 (neutral)
-
Question: How many protons, neutrons, and electrons are in the ion 39K+?
Answer:- Protons: 19
- Neutrons: 20
- Electrons: 18
-
Question: Define the term “isotope” and give an example.
Answer:- Definition: Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons, resulting in different mass numbers.
- Example: Carbon-12 and Carbon-14
-
Question: Calculate the average atomic mass of element X if it has two isotopes: X-20 (50%) and X-22 (50%).
Answer:- Average Atomic Mass: (0.50 * 20 amu) + (0.50 * 22 amu) = 21 amu
-
Question: Write the electron configuration for Oxygen (O).
Answer:- Electron Configuration: 1s22s22p4
-
Question: What is the difference between atomic number and mass number?
Answer:- Atomic Number: The number of protons in the nucleus of an atom. It defines the element.
- Mass Number: The total number of protons and neutrons in the nucleus of an atom.
-
Question: What is an ion? Describe how a cation and an anion are formed.
Answer:- Ion: An atom or molecule with a net electric charge due to the loss or gain of electrons.
- Cation: A positively charged ion formed when an atom loses electrons.
- Anion: A negatively charged ion formed when an atom gains electrons.
-
Question: Describe the location of protons, neutrons, and electrons in an atom.
Answer:- Protons: Located in the nucleus.
- Neutrons: Located in the nucleus.
- Electrons: Orbit the nucleus in electron shells or energy levels.
-
Question: What is the significance of valence electrons?
Answer:- Valence electrons are the electrons in the outermost shell of an atom. They determine the chemical properties of the element and how it will interact with other atoms to form chemical bonds.
-
Question: Define the terms: a) Ground state, b) Excited state
Answer:- Ground State: The lowest energy state of an atom.
- Excited State: Any energy state of an atom that is higher than the ground state. Electrons can be excited to higher energy levels by absorbing energy.
Remember to review your notes and textbook for a more in-depth understanding of these concepts. Use these answers as a guide to check your work and identify areas where you need further review. Good luck with your studies!
If you are searching about Atomic Structure Worksheet Answer – Printable And Enjoyable Learning you’ve visit to the right place. We have 20 Pics about Atomic Structure Worksheet Answer – Printable And Enjoyable Learning like Atomic Structure Review Worksheet – E-streetlight.com, Atomic structure wkst – science – Worksheet: Atomic Structure and also Tes Atomic Structure Worksheet. Read more:
Atomic Structure Worksheet Answer – Printable And Enjoyable Learning

newark2.remotepc.com
Periodic Table Review Worksheet Luxury Atomic Structure Review

chessmuseum.org
Atomic Structure Worksheet

ar.inspiredpencil.com
Atomic Structure Worksheet – Studocu – Worksheets Library

worksheets.clipart-library.com
Atomic Structure Worksheet Table

nasreen-nasbloggie.blogspot.com
Atomic Structure Wkst – Science – Worksheet: Atomic Structure
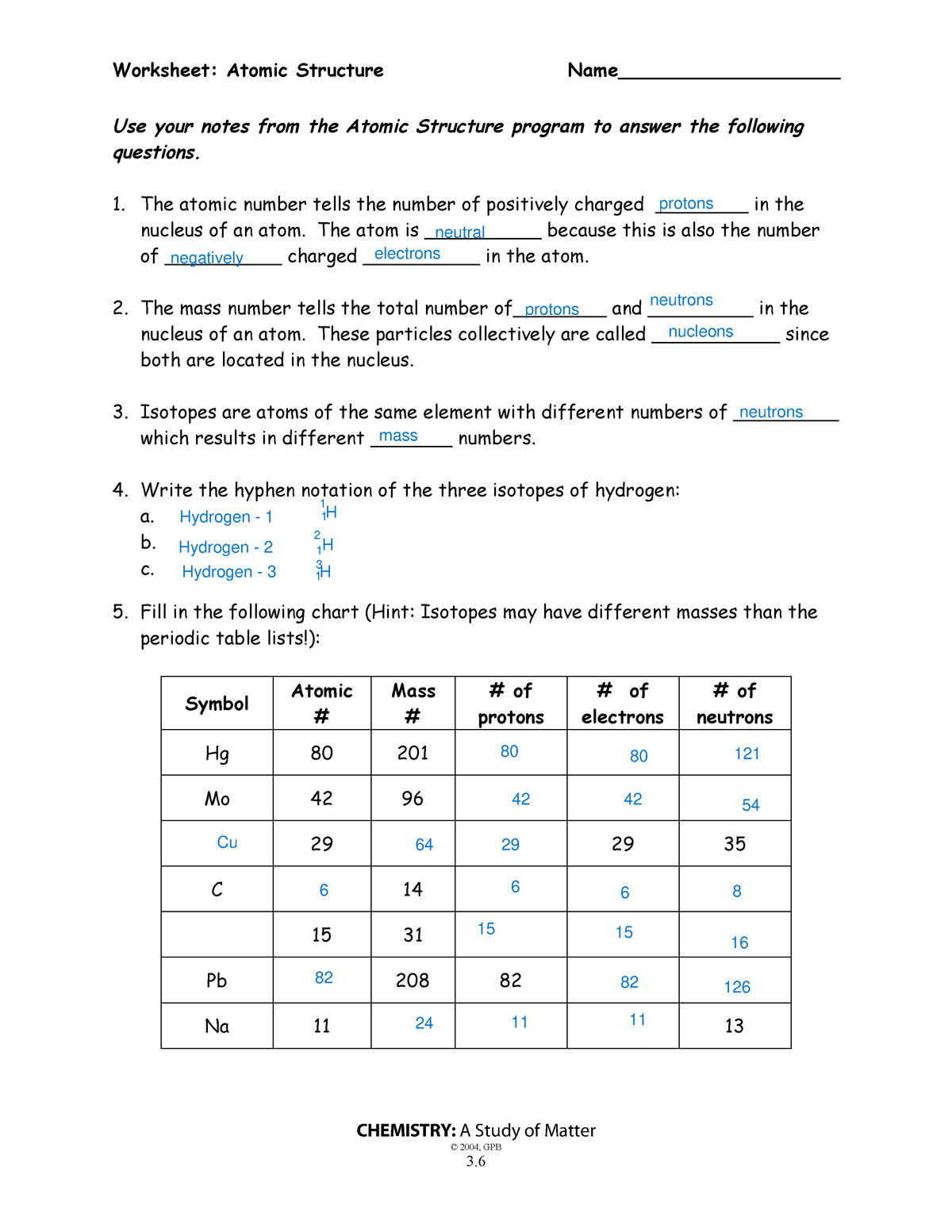
worksheets.clipart-library.com
Atomic Structure Review Worksheet – Printable Word Searches
davida.davivienda.com
Atomic Structure Worksheet Answers – E-streetlight.com
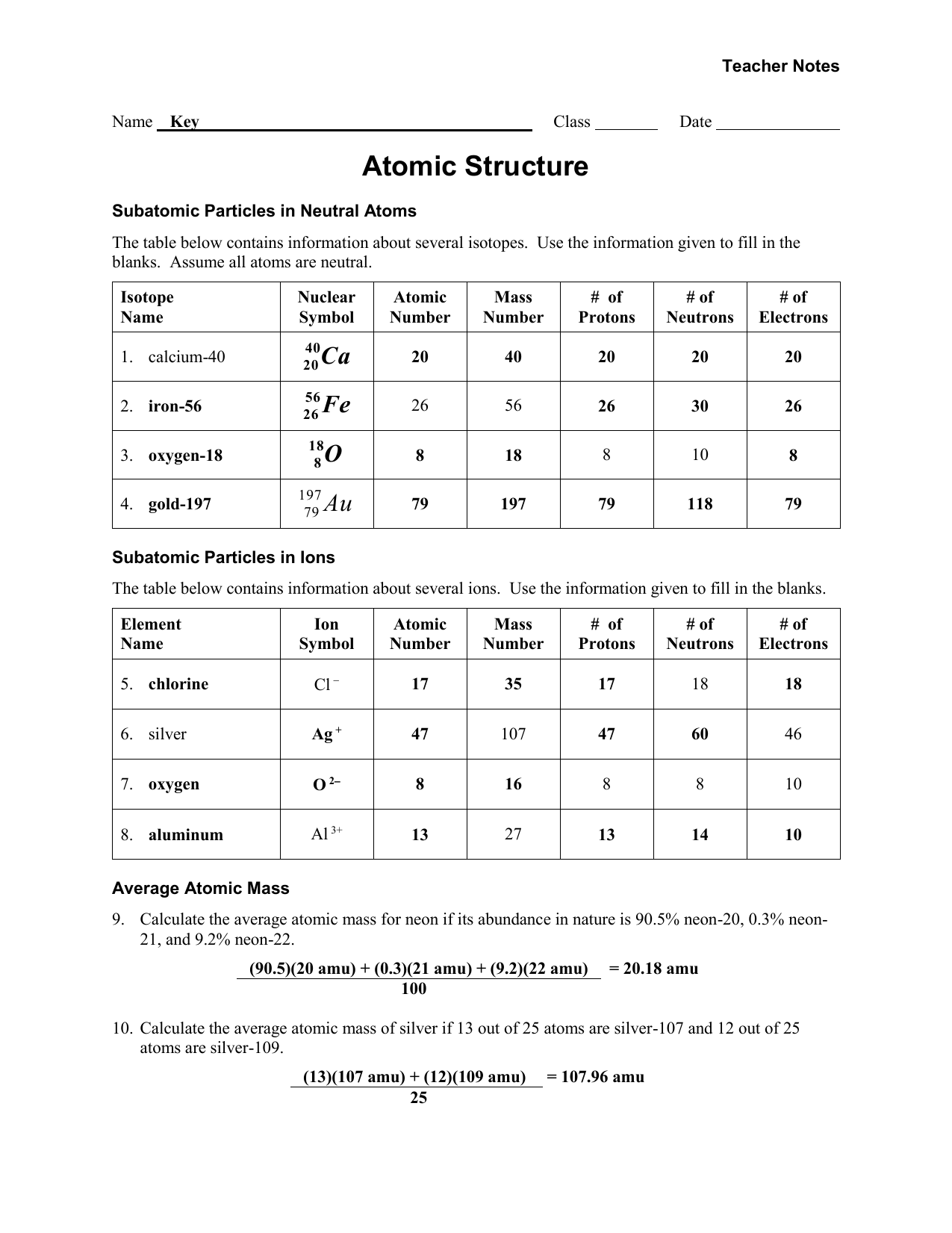
www.e-streetlight.com
Atomic Structure Worksheet Answer Key – Proworksheet.my.id
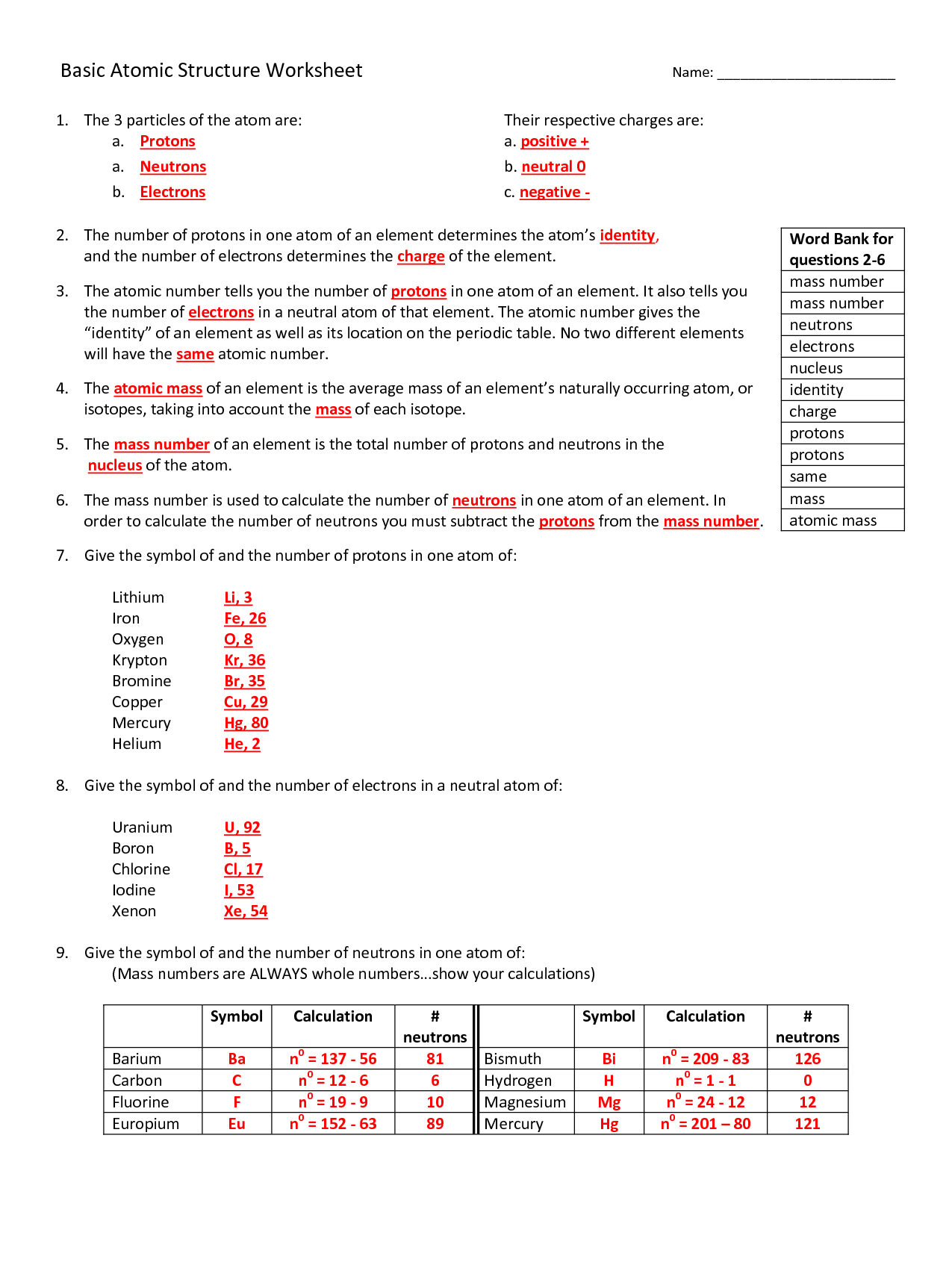
www.proworksheet.my.id
Atomic Structure Review Worksheet – E-streetlight.com
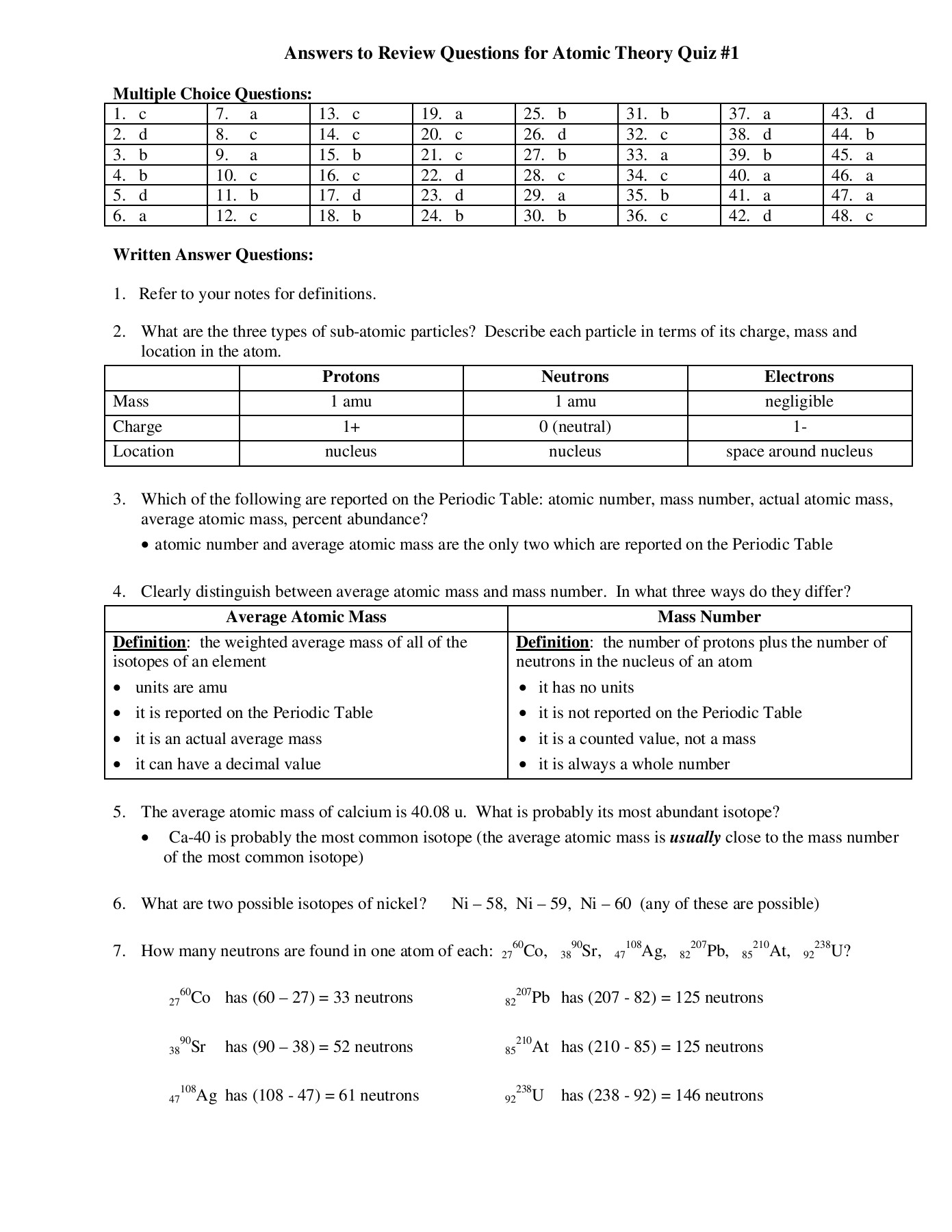
www.e-streetlight.com
Drawing Atoms Worksheet Answer Key Lovely Lewis Dot Structures

www.pinterest.co.uk
Worksheet Atomic Structure

esquintan3nwdblearning.z14.web.core.windows.net
Basic Atomic Structure Worksheet

learningschoolportulacq4.z22.web.core.windows.net
Atomic Structure Worksheet Pdf – Pro Worksheet

www.proworksheet.my.id
Atomic Structure Worksheet
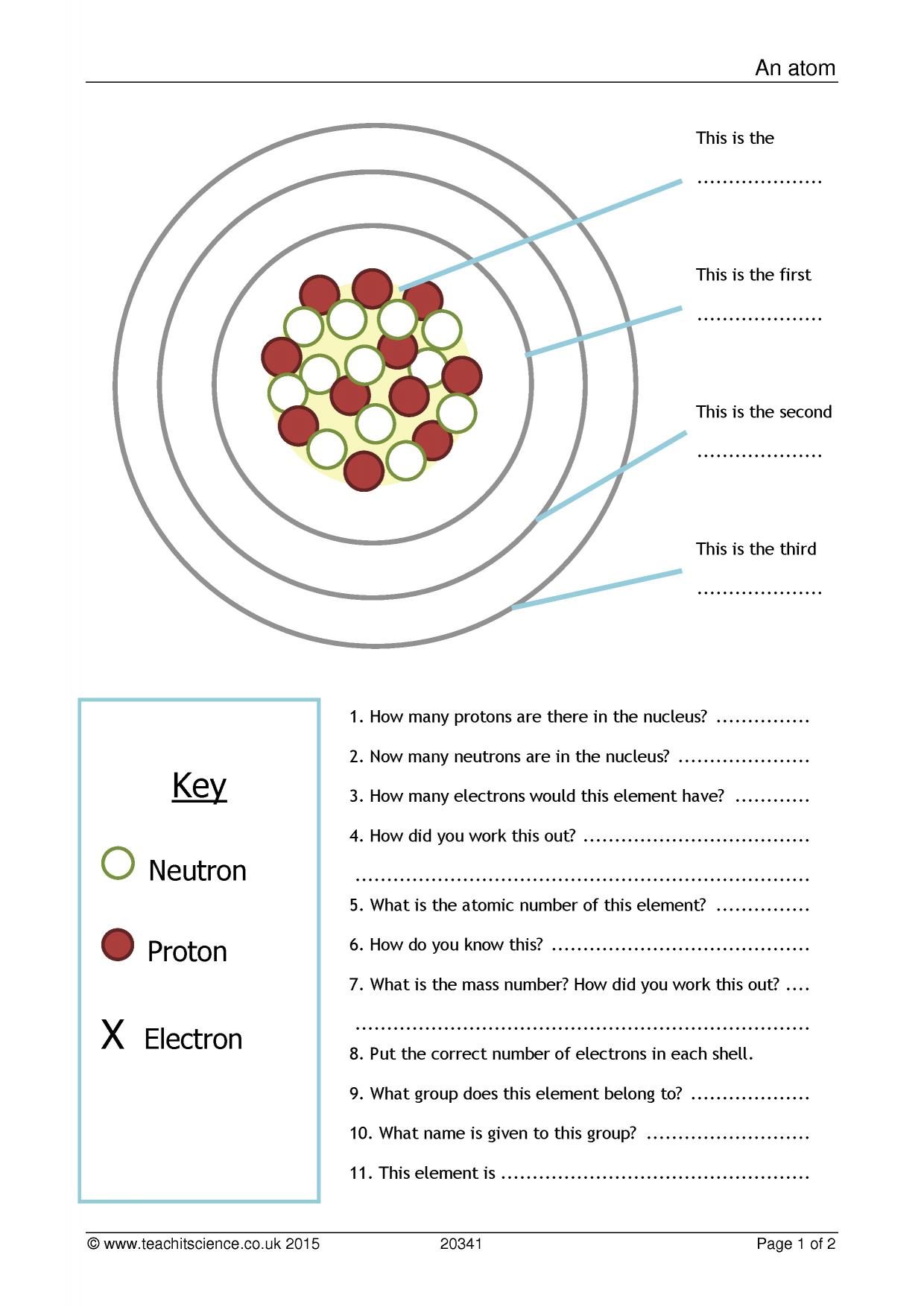
ar.inspiredpencil.com
Atomic Structure Worksheet
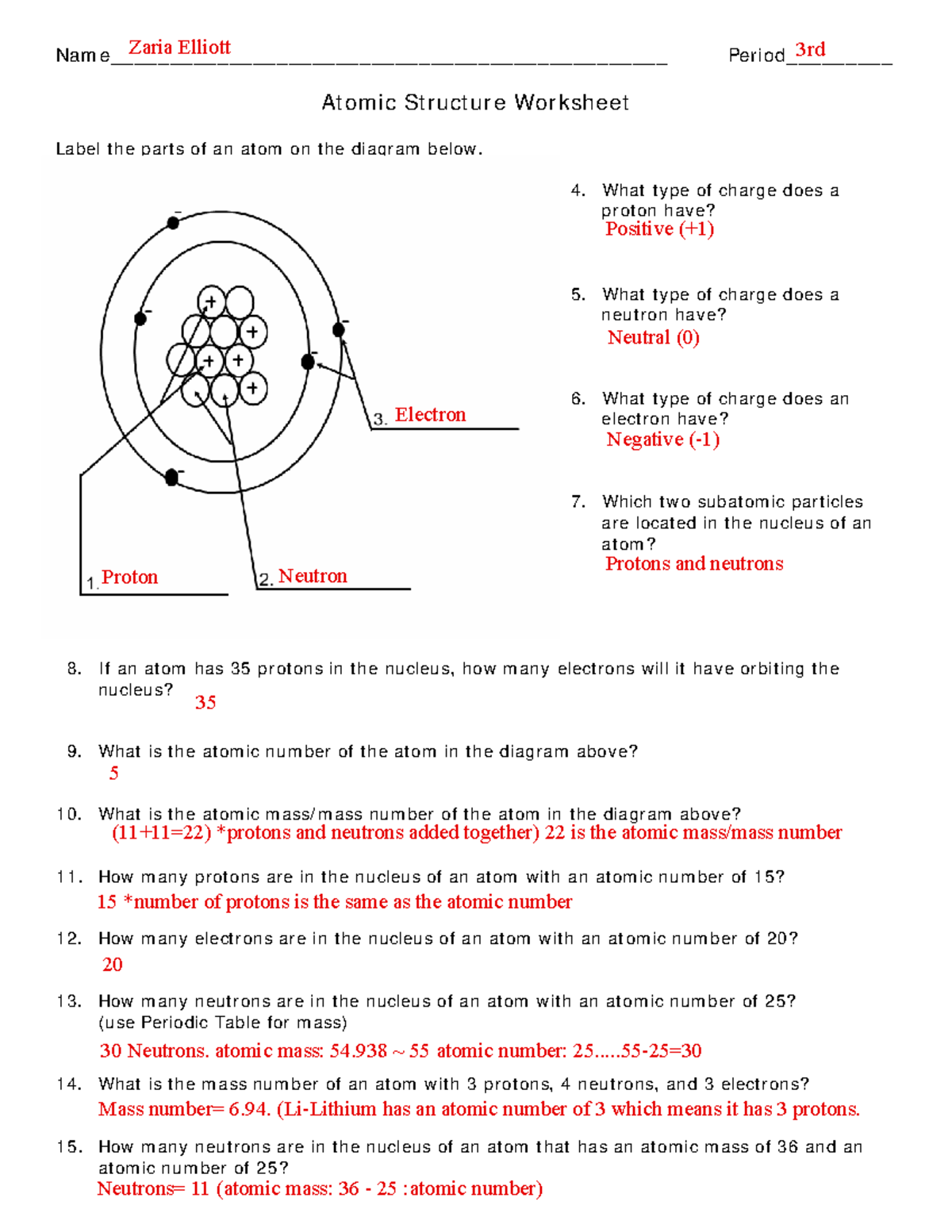
ar.inspiredpencil.com
Tes Atomic Structure Worksheet

studyescoliy9.z21.web.core.windows.net
Atomic Structure Review Sheet With Answers | Exercises Chemistry

worksheets.clipart-library.com
Unit Atomic Structure Worksheets Answers
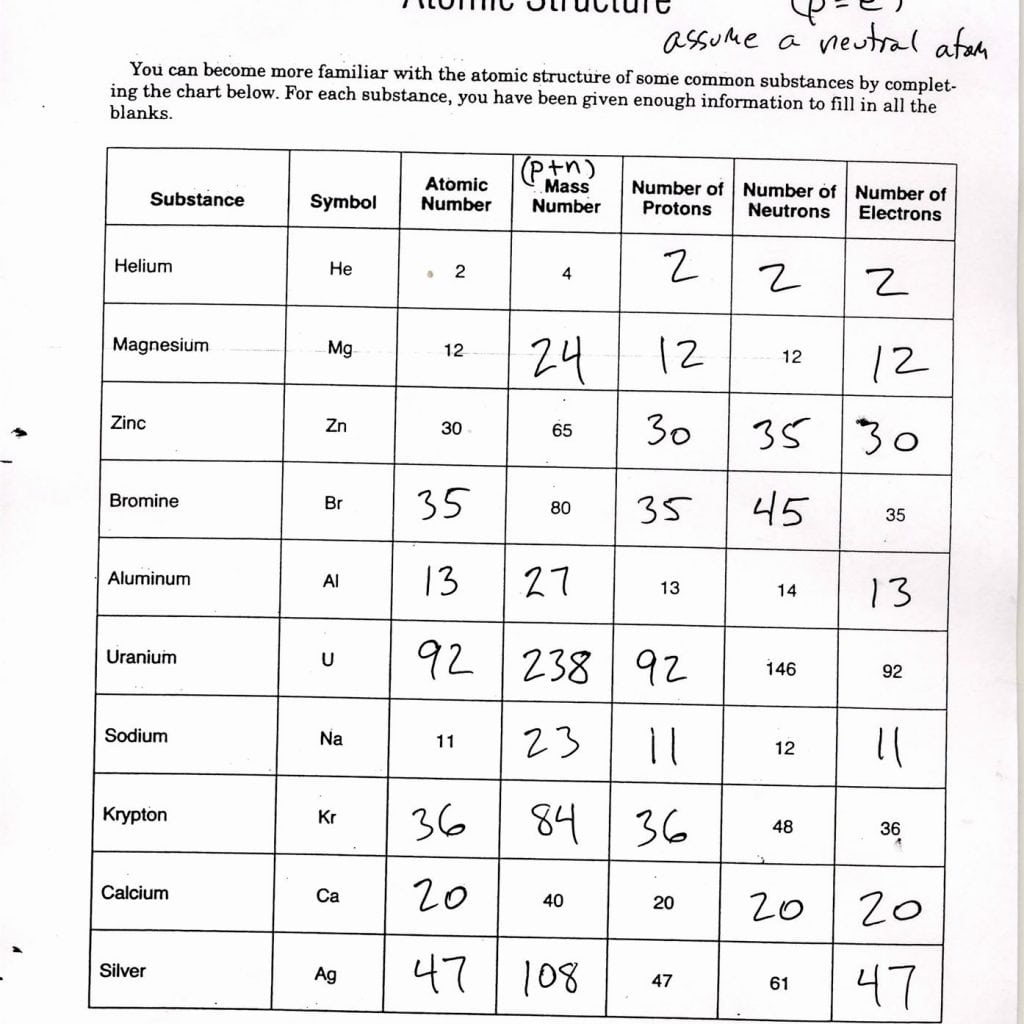
learningzoneppquartyj.z21.web.core.windows.net
Atomic Structure Review Worksheet Answers – Printable Word Searches

davida.davivienda.com
Atomic structure worksheet answer. Atomic structure review worksheet – e-streetlight.com. Atomic structure worksheet answer key