Struggling with that Bohr Atomic Models worksheet? You’re not alone! Understanding the Bohr model is a crucial step in grasping the fundamentals of atomic structure and quantum mechanics. It lays the groundwork for understanding more complex atomic theories and behaviors. This worksheet typically covers topics like calculating energy levels, determining wavelengths of emitted light, and understanding the relationship between electron transitions and spectral lines. It can be a bit daunting, especially when first introduced to the concept of quantized energy and the idea that electrons can only exist in specific orbits.
The Bohr model, while simplified compared to modern quantum mechanical models, provides a valuable visual representation of how electrons orbit the nucleus and how these orbits are associated with specific energy levels. Remember, electrons don’t just orbit randomly; they can only exist in specific “shells” or energy levels, labeled as n=1, n=2, n=3, and so on. When an electron jumps from a higher energy level to a lower energy level, it emits a photon of light with a specific wavelength and energy. The Bohr model allows us to calculate these energy differences and predict the wavelengths of emitted light.
This post is designed to provide you with the answers to a common Bohr Atomic Models worksheet, along with a brief explanation to help you understand the underlying concepts. It’s important to note that simply copying the answers won’t lead to true understanding. Instead, use these answers as a guide to check your work and identify areas where you might need further review. Make sure you understand the formulas used, the relationship between energy levels and wavelengths, and the implications of electron transitions. With a bit of effort, you’ll be mastering the Bohr model in no time!
Bohr Atomic Models Worksheet Answers
Below you will find the answers to a typical Bohr Atomic Models Worksheet. Remember to use these answers as a tool to check your work and deepen your understanding. Let’s break down some common questions and their solutions:
Disclaimer: Worksheet questions and numbers can vary slightly. These answers are representative of a common type of Bohr Atomic Model worksheet. Please confirm that your worksheet aligns with the question types provided below.
Section 1: Energy Level Calculations
- Question 1: Calculate the energy of an electron in the n=1 energy level of hydrogen.
- Answer:
- Formula: En = -13.6 eV / n2
- E1 = -13.6 eV / (1)2 = -13.6 eV
- Question 2: Calculate the energy of an electron in the n=3 energy level of hydrogen.
- Answer:
- Formula: En = -13.6 eV / n2
- E3 = -13.6 eV / (3)2 = -13.6 eV / 9 = -1.51 eV (approximately)
- Question 3: What is the energy difference between the n=2 and n=4 energy levels in hydrogen?
- Answer:
- First, calculate E2 and E4
- E2 = -13.6 eV / (2)2 = -13.6 eV / 4 = -3.4 eV
- E4 = -13.6 eV / (4)2 = -13.6 eV / 16 = -0.85 eV
- ΔE = E4 – E2 = -0.85 eV – (-3.4 eV) = 2.55 eV
Section 2: Wavelength and Frequency Calculations
- Question 4: An electron in a hydrogen atom transitions from n=3 to n=2. Calculate the wavelength of the emitted photon.
- Answer:
- First, calculate the energy difference (ΔE).
- E2 = -3.4 eV (from previous question)
- E3 = -1.51 eV (from previous question)
- ΔE = E2 – E3 = -3.4 eV – (-1.51 eV) = -1.89 eV (Note: The negative sign indicates energy is emitted)
- Convert eV to Joules: -1.89 eV * 1.602 x 10-19 J/eV = -3.028 x 10-19 J
- Use the equation: E = hc/λ, where h = Planck’s constant (6.626 x 10-34 J s), c = speed of light (3.0 x 108 m/s)
- λ = hc/E = (6.626 x 10-34 J s * 3.0 x 108 m/s) / (3.028 x 10-19 J) = 6.57 x 10-7 m or 657 nm
- Question 5: Calculate the frequency of the photon emitted in the transition described in question 4.
- Answer:
- Use the equation: E = hf, where h = Planck’s constant (6.626 x 10-34 J s)
- f = E/h = (3.028 x 10-19 J) / (6.626 x 10-34 J s) = 4.57 x 1014 Hz
Section 3: Bohr Model Concepts
- Question 6: Explain why the Bohr model is considered a “quantized” model of the atom.
- Answer: The Bohr model is considered quantized because it postulates that electrons can only exist in specific, discrete energy levels or orbits around the nucleus. Electrons cannot exist in between these levels. Each energy level corresponds to a specific allowed radius for the electron’s orbit. This is in contrast to classical physics, which would allow for a continuous range of energy levels and orbital radii.
- Question 7: What happens when an electron absorbs a photon in the Bohr model?
- Answer: When an electron absorbs a photon with energy equal to the difference between two allowed energy levels, the electron will jump to a higher energy level (a level further from the nucleus). This is called excitation. The electron will then eventually fall back down to a lower energy level, releasing the absorbed energy as a photon of light.
- Question 8: What are the limitations of the Bohr model?
- Answer: The Bohr model has several limitations:
- It only accurately predicts the spectra of hydrogen and other single-electron ions.
- It fails to explain the spectra of more complex atoms.
- It violates the Heisenberg uncertainty principle.
- It assumes that electrons travel in fixed orbits, which is not accurate according to modern quantum mechanics.
- It does not explain the fine structure or hyperfine structure of spectral lines.
Hopefully, these answers and explanations will help you better understand the Bohr Atomic Model. Remember to practice similar problems and review the underlying concepts to solidify your knowledge. Good luck!
If you are looking for Bohr Atomic Models Worksheet | Worksheets, Cursive writing worksheets you’ve came to the right page. We have 20 Pics about Bohr Atomic Models Worksheet | Worksheets, Cursive writing worksheets like Bohr atomic Models Worksheet Answers Luxury 18 Best Of Bohr Diagram, Bohr atomic models worksheet answers worksheet for education – Artofit and also Bohr Atomic Models Worksheet – E-streetlight.com. Here you go:
Bohr Atomic Models Worksheet | Worksheets, Cursive Writing Worksheets

www.pinterest.com
Bohr Atomic Models Worksheet Answers – Pro Worksheet
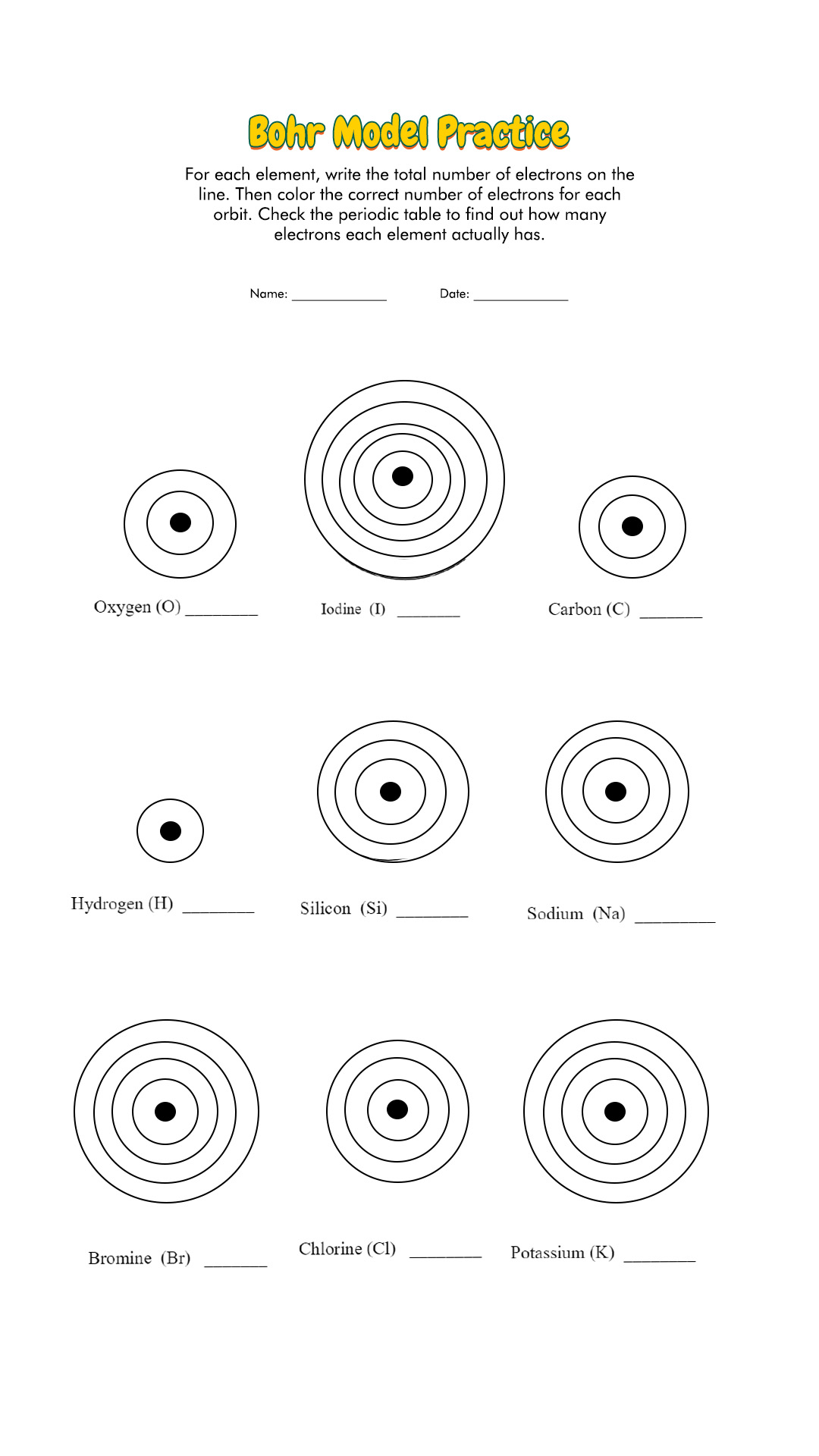
www.proworksheet.my.id
Bohr Atomic Models Worksheet Answers – Pro Worksheet

www.proworksheet.my.id
Bohr Model Worksheet Form ≡ Fill Out Printable PDF Forms Online
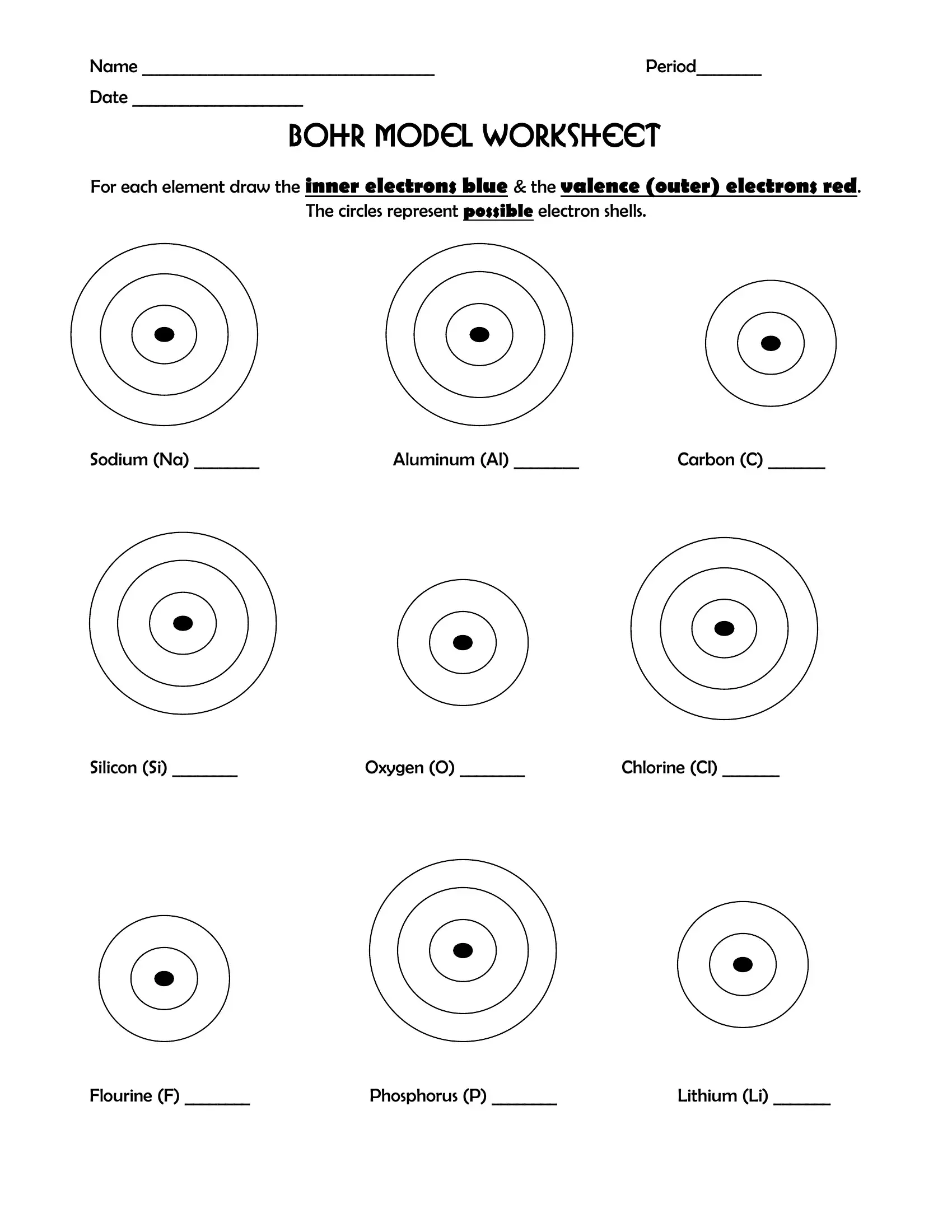
formspal.com
Bohr Atomic Models Worksheet Answers Worksheet For Education – Artofit

www.artofit.org
Bohr Atomic Models Worksheet Answers Worksheets Bohr Model Worksheet

br.pinterest.com
Bohr Model Practice – Chemistry Periodic Table – Name: Date

worksheets.clipart-library.com
Bohr Atomic Models Worksheet – Pro Worksheet
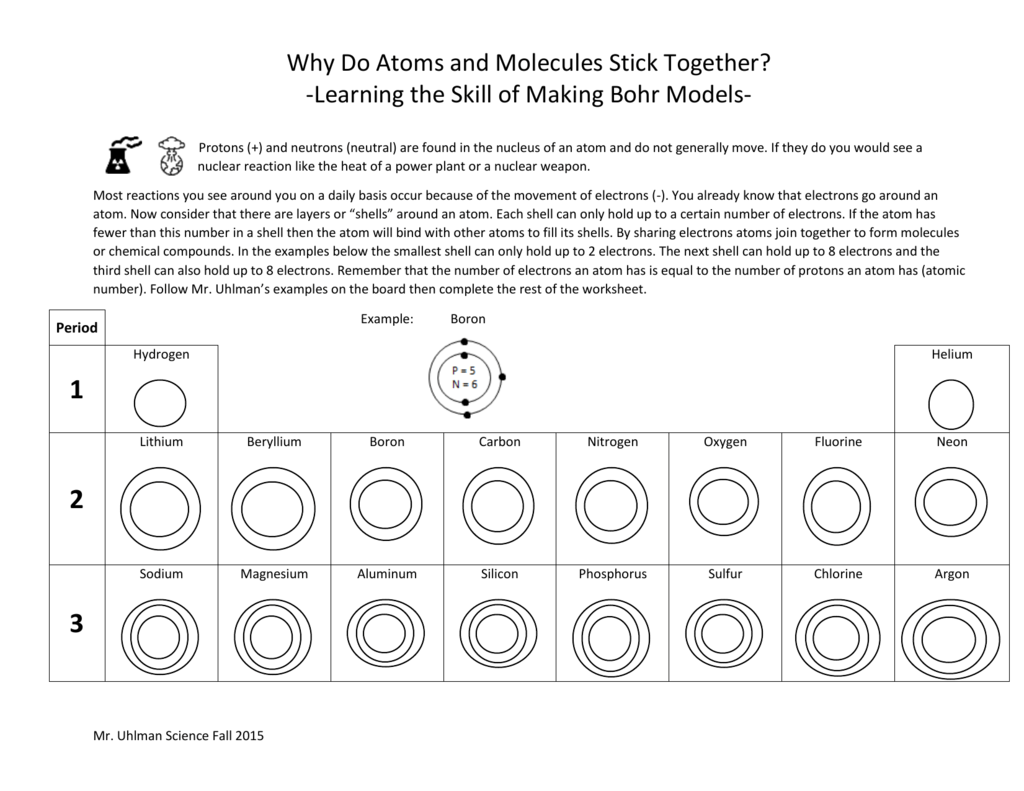
www.proworksheet.my.id
Worksheet Atomic Structure Answers – E-streetlight.com
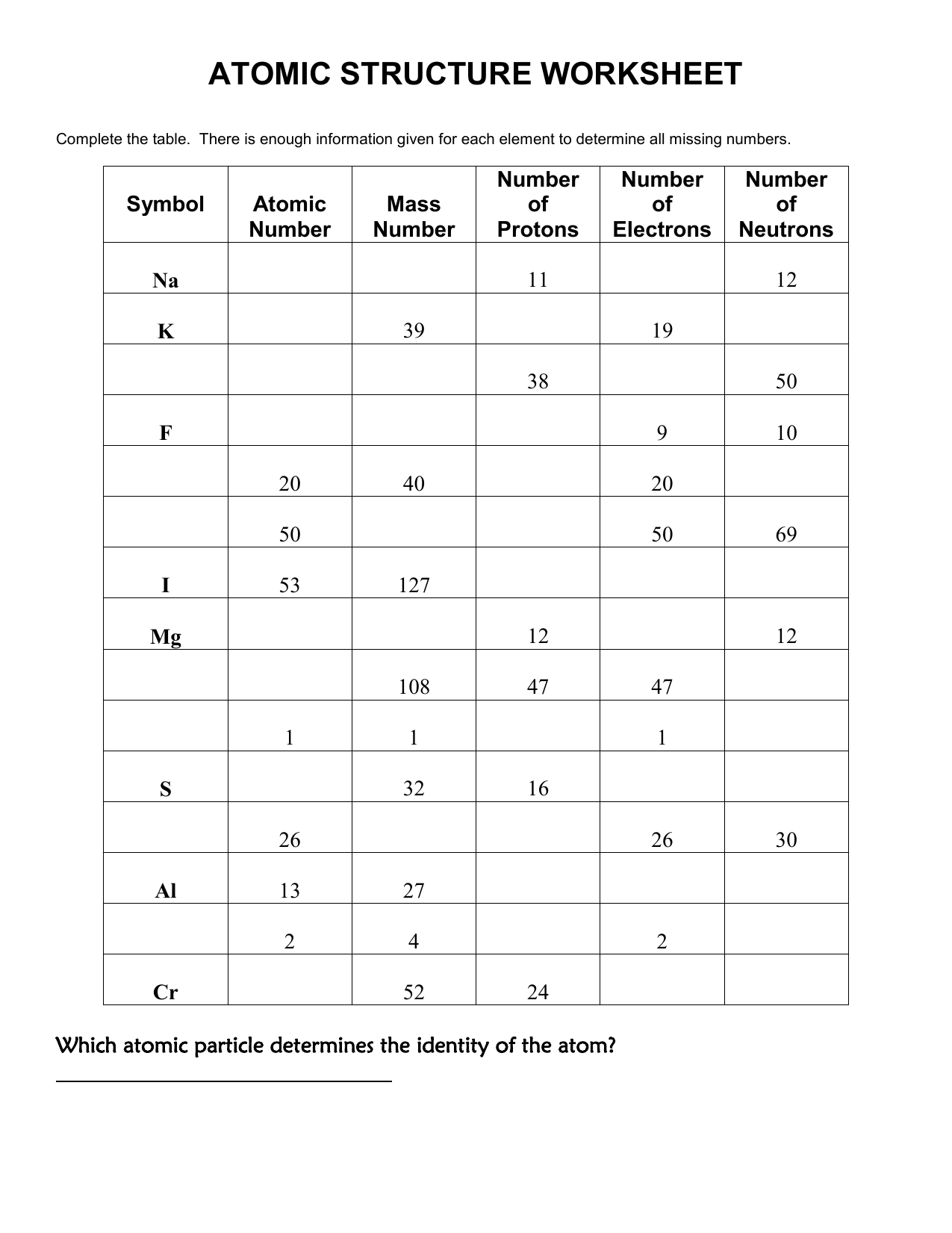
www.e-streetlight.com
Models Of The Atom Worksheet Answers – Printable PDF Template

martinlindelof.com
Bohr Atomic Models Worksheet – Printable Word Searches

davida.davivienda.com
Bohr Model Worksheet Answers – Pro Worksheet
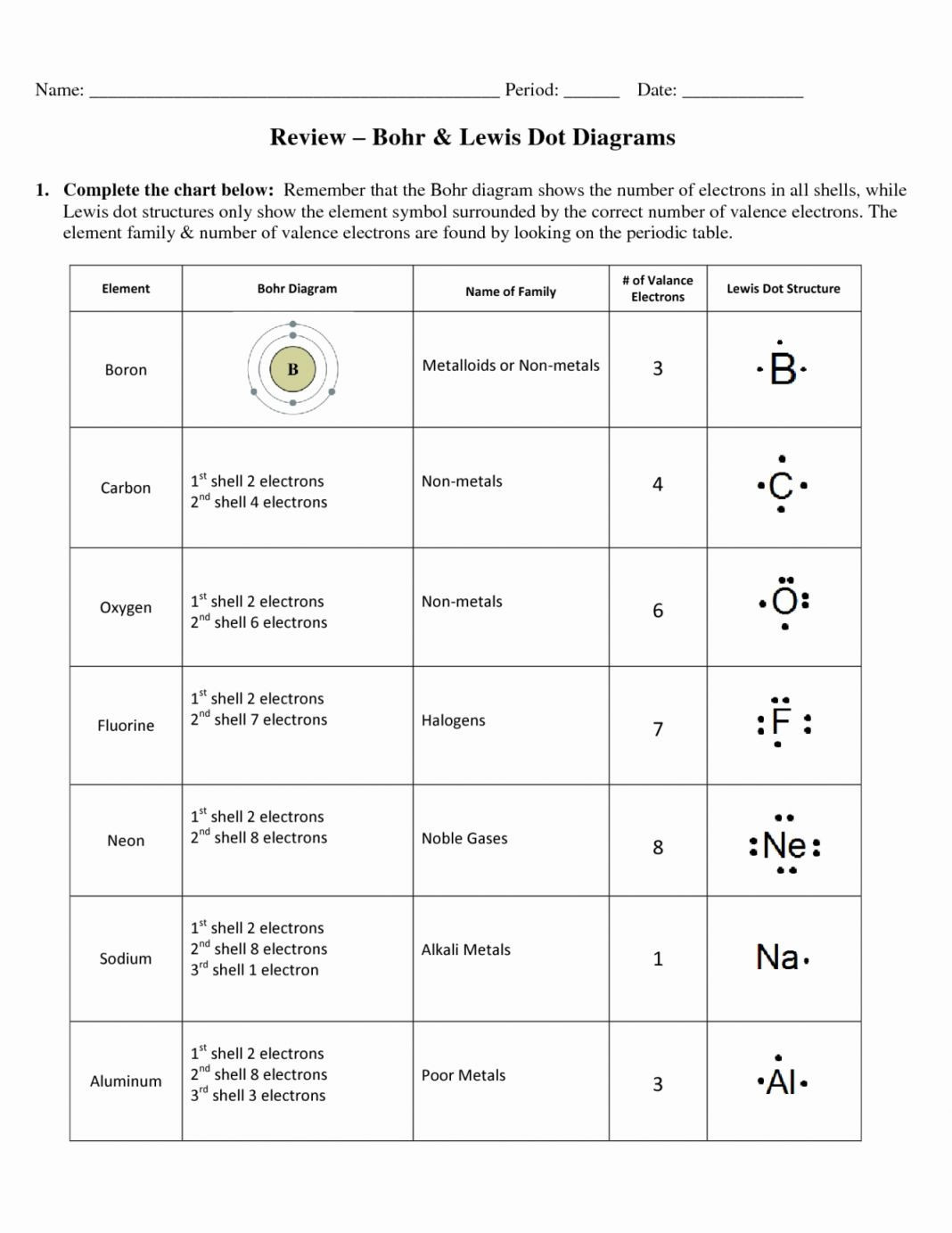
www.proworksheet.my.id
Bohr Atomic Models Worksheet – E-streetlight.com
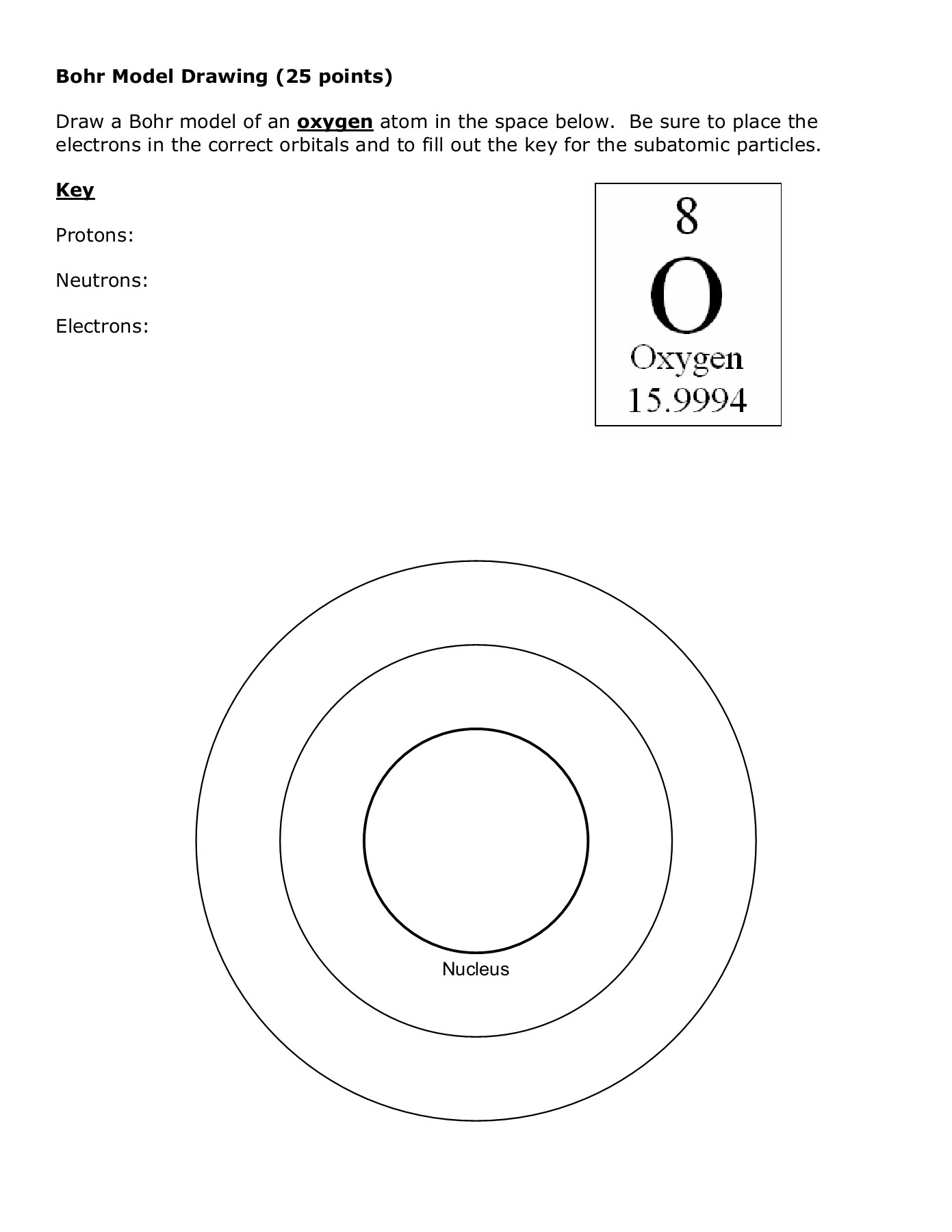
www.e-streetlight.com
Bohr Atomic Models Worksheet – E-streetlight.com

www.e-streetlight.com
Bohr Atomic Models Worksheet Answers

www.pinterest.com
Bohr Model Practice Worksheet – Printable Word Searches

davida.davivienda.com
Bohr Atomic Models Worksheet Answers Luxury 18 Best Of Bohr Diagram
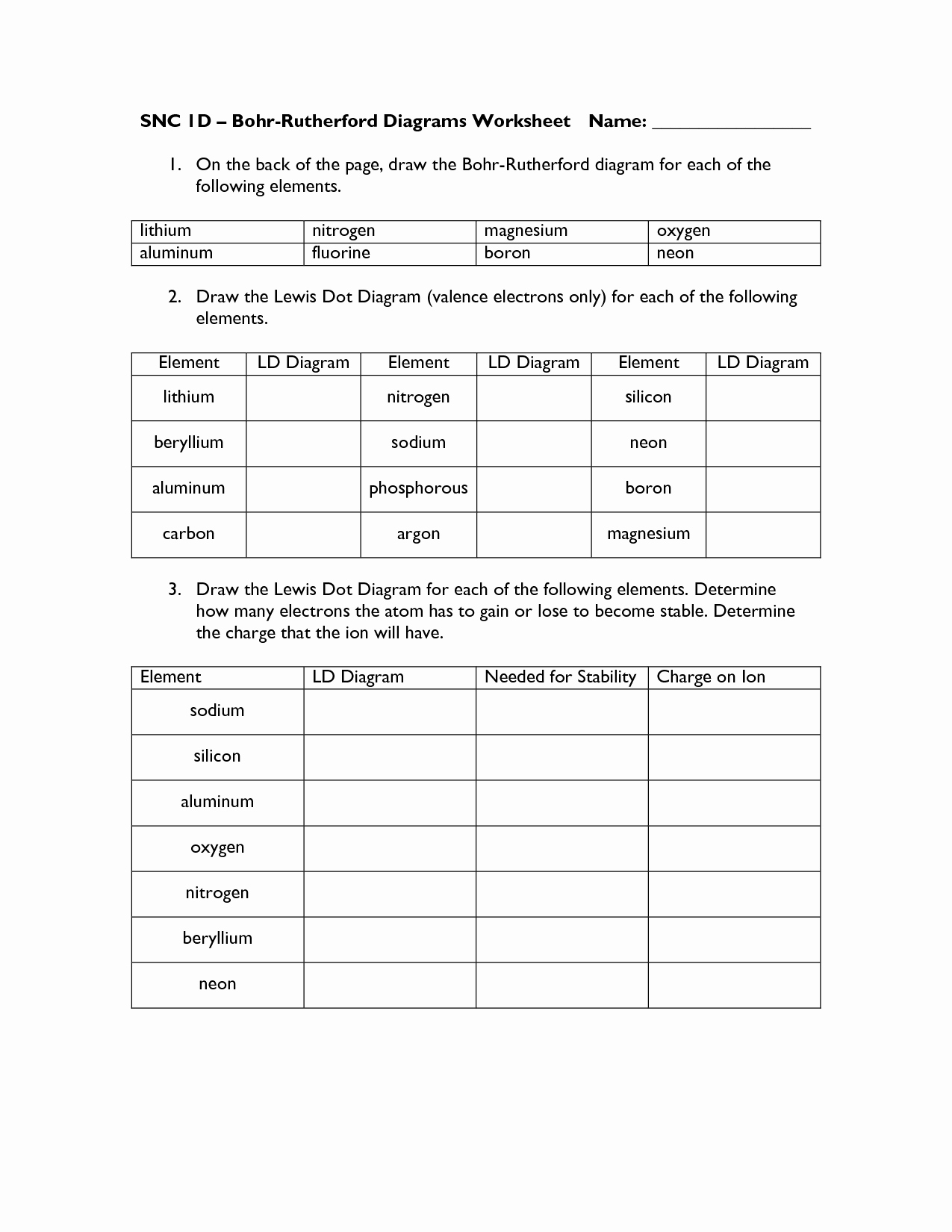
chessmuseum.org
Bohr Atomic Models Worksheet Awesome Bohr Model Worksheet Key
chessmuseum.org
Bohr Atomic Model Worksheet Answer Key – Parleyinspire

parleyinspire.blogspot.com
Bohr Model Worksheet Answers – Printable Word Searches

davida.davivienda.com
bohr atomic models worksheet answers – pro worksheet. bohr model practice. Bohr atomic models worksheet awesome bohr model worksheet key