Struggling with Gibbs Free Energy and how it dictates the spontaneity of reactions? You’re not alone! This crucial thermodynamic concept can be tricky to grasp, especially when dealing with enthalpy, entropy, and temperature all playing their roles. To help you conquer this hurdle, we’ve prepared a comprehensive Gibbs Free Energy worksheet, complete with practice problems designed to solidify your understanding. Whether you’re a high school chemistry student, an undergraduate science major, or simply looking to brush up on your thermodynamics, this worksheet is a valuable resource. It provides a structured approach to tackling various Gibbs Free Energy calculations and interpreting the results to predict reaction spontaneity.
The Gibbs Free Energy (G) combines enthalpy (H), which represents the heat content of a system, and entropy (S), which quantifies the disorder or randomness of the system. The relationship is elegantly expressed by the equation: G = H – TS, where T is the temperature in Kelvin. A negative value of ΔG (the change in Gibbs Free Energy) indicates a spontaneous (or favorable) reaction under the given conditions. A positive ΔG signifies a non-spontaneous reaction, requiring external energy input to proceed. And a ΔG of zero indicates that the reaction is at equilibrium.
Our worksheet covers a variety of problem types, including calculating ΔG from ΔH and ΔS, determining the temperature at which a reaction becomes spontaneous, and relating ΔG to the equilibrium constant K. Each problem is carefully crafted to challenge your understanding and reinforce the fundamental principles of Gibbs Free Energy. Remember to pay close attention to units, signs, and the impact of temperature on spontaneity.
Gibbs Free Energy Worksheet Answers
Below are the answers to the Gibbs Free Energy Worksheet. We encourage you to attempt the problems before checking the solutions. Use these answers as a guide to check your work and identify areas where you may need further practice. Understanding *how* to arrive at the correct answer is more important than just getting the answer itself, so be sure to review your calculations and reasoning.
Worksheet Answers:
- Problem 1: ΔG = -285.8 kJ/mol
- Problem 2: Spontaneous at all temperatures
- Problem 3: T = 373 K
- Problem 4: ΔG = -57.2 kJ/mol
- Problem 5: Non-spontaneous at 298 K; spontaneous at high temperatures. Need to calculate T where ΔG=0, so T = ΔH/ΔS
- Problem 6: K = 6.66 x 105
- Problem 7: ΔG = -20.6 kJ/mol
-
Problem 8:
- ΔH = -198.4 kJ
- ΔS = -187.9 J/K
- ΔG = -142.4 kJ (Spontaneous)
-
Problem 9:
- ΔH = +131 kJ/mol
- ΔS = +133 J/mol·K
- Spontaneous at high temperatures. Calculation requires T = ΔH/ΔS = 985 K
-
Problem 10:
- ΔG = +210.7 kJ/mol
- Reaction is not spontaneous under standard conditions.
- To make the reaction spontaneous we could increase the temperature.
Explanation of key concepts:
Spontaneity: A negative ΔG indicates a spontaneous process, meaning it will proceed without external energy input. A positive ΔG indicates a non-spontaneous process, requiring energy to proceed. A ΔG of zero signifies equilibrium.
Temperature Dependence: The spontaneity of a reaction can be temperature-dependent, especially when ΔH and ΔS have the same sign. A reaction might be non-spontaneous at low temperatures but spontaneous at higher temperatures, or vice versa. The temperature at which the reaction switches spontaneity can be calculated by setting ΔG = 0 and solving for T (T = ΔH/ΔS).
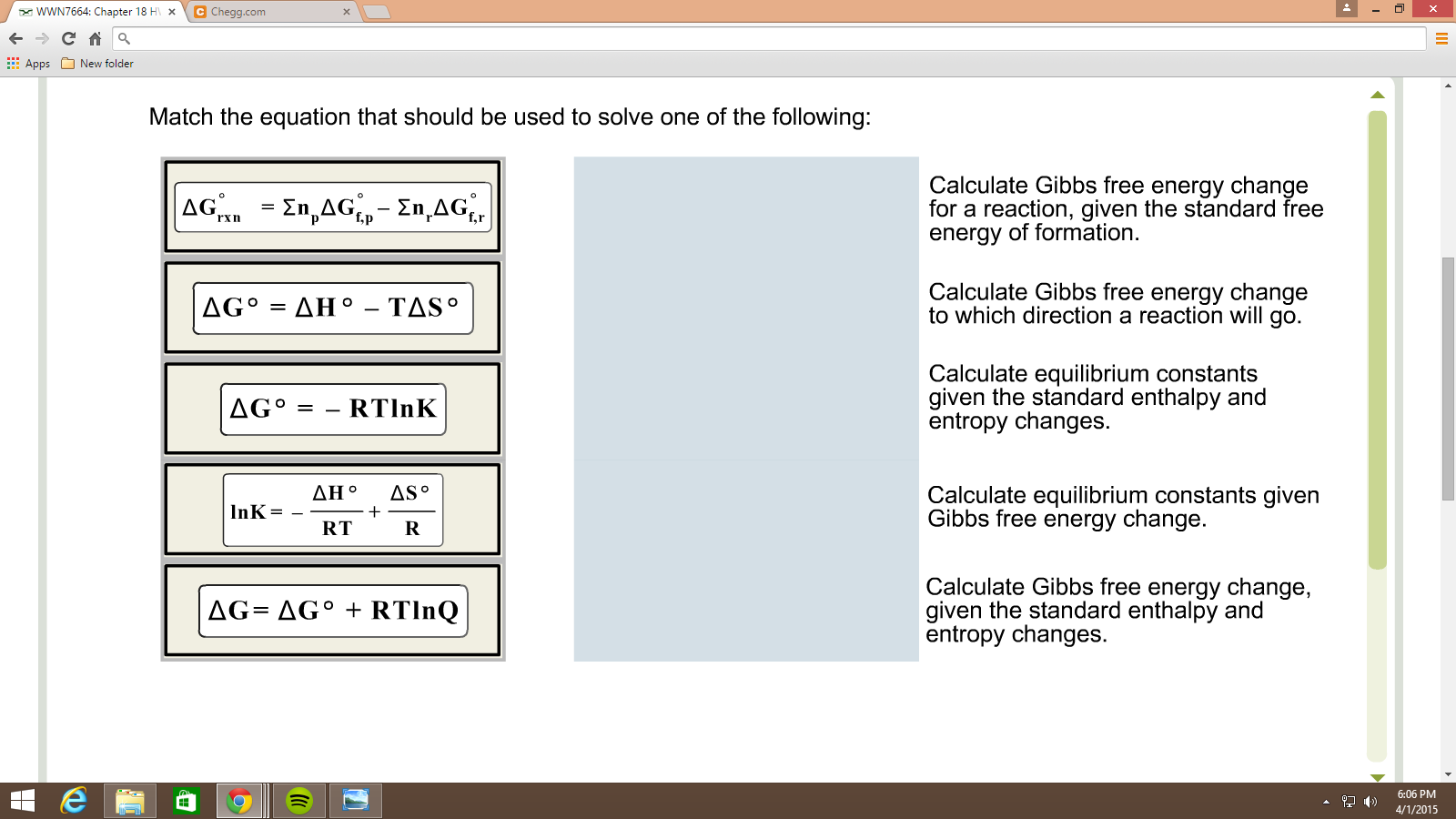
Equilibrium Constant (K): The Gibbs Free Energy is related to the equilibrium constant by the equation ΔG = -RTlnK, where R is the gas constant and T is the temperature in Kelvin. A large K indicates that the products are favored at equilibrium, and a small K indicates that the reactants are favored.
Remember to review the definitions of enthalpy and entropy. Enthalpy is the measure of the heat content of a system at constant pressure. Exothermic reactions (releasing heat) have negative ΔH values, while endothermic reactions (absorbing heat) have positive ΔH values. Entropy is a measure of disorder or randomness within a system. Processes that increase disorder have positive ΔS values, and processes that decrease disorder have negative ΔS values. Use these principles alongside the Gibbs Free Energy equation to predict the spontaneity and equilibrium of chemical reactions. Good luck with your studies!
If you are searching about Free work energy and power worksheet answers, Download Free work energy you’ve visit to the right page. We have 20 Pics about Free work energy and power worksheet answers, Download Free work energy like Gibbs Free Energy Worksheet Unique Quiz & Worksheet Spontaneous, Gibbs Free Energy Worksheet – Pro Worksheet and also Gibbs Free Energy Worksheet – Pro Worksheet. Read more:
Free Work Energy And Power Worksheet Answers, Download Free Work Energy

worksheets.clipart-library.com
Graphs Of Gibbs Free Energy

www.chemca.in
Gibbs Free Energy Formula Stock Vector (Royalty Free) 2291204789

www.shutterstock.com
Gibbs Free Energy Worksheet – Pro Worksheet

www.proworksheet.my.id
SOLUTION: Chapter 17 Gibbs Free Energy Worksheet Winter 2024 1 – Studypool

www.studypool.com
15 Physics Work Energy And Power Worksheet – Free PDF At

worksheets.clipart-library.com
Gibbs Free Energy Worksheet – Pro Worksheet
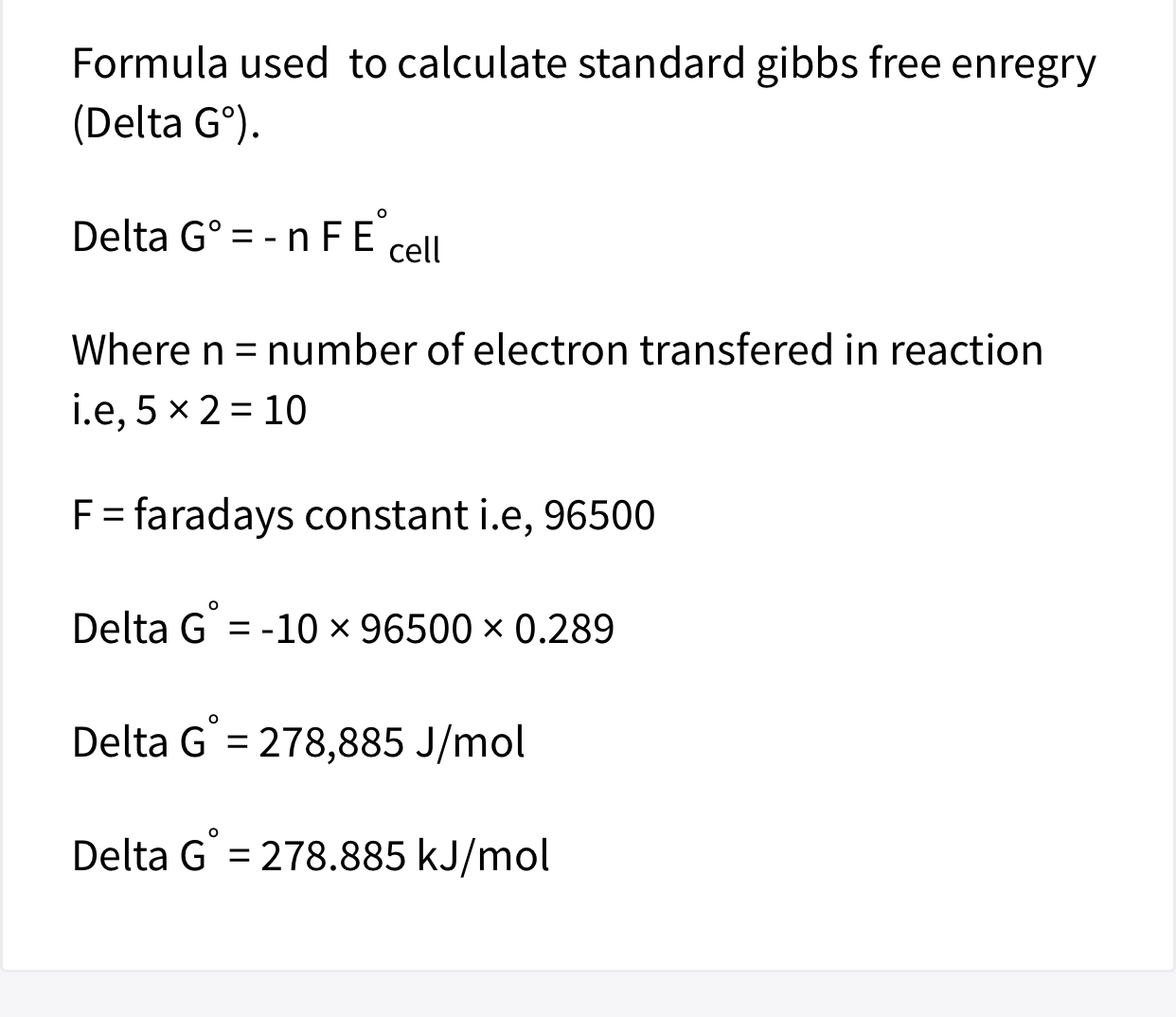
www.proworksheet.my.id
Gibbs Free Energy Worksheet – E-streetlight.com

www.e-streetlight.com
Gibbs Free Energy | Science, Chemistry | ShowMe – Worksheets Library

worksheets.clipart-library.com
Types Of Energy Worksheet – Pro Worksheet
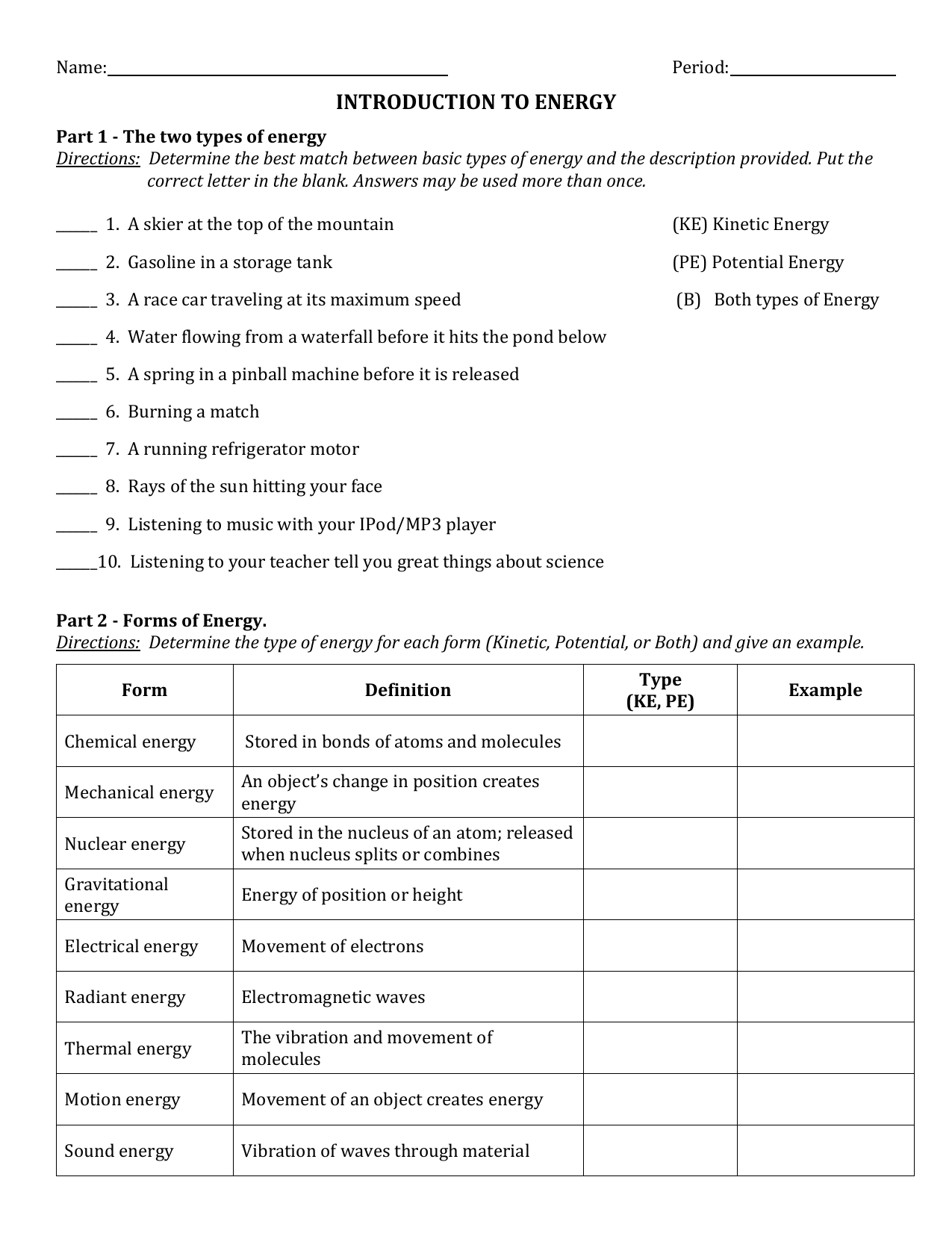
www.proworksheet.my.id
Gibbs Free Energy Worksheets

learninglibrarysteiner.z21.web.core.windows.net
Forms Of Energy Worksheet: Free Printable PDF For Children – Worksheets

worksheets.clipart-library.com
Free Energy Worksheet Answers, Download Free Energy Worksheet Answers

worksheets.clipart-library.com
Gibbs Free Energy Worksheet – E-streetlight.com
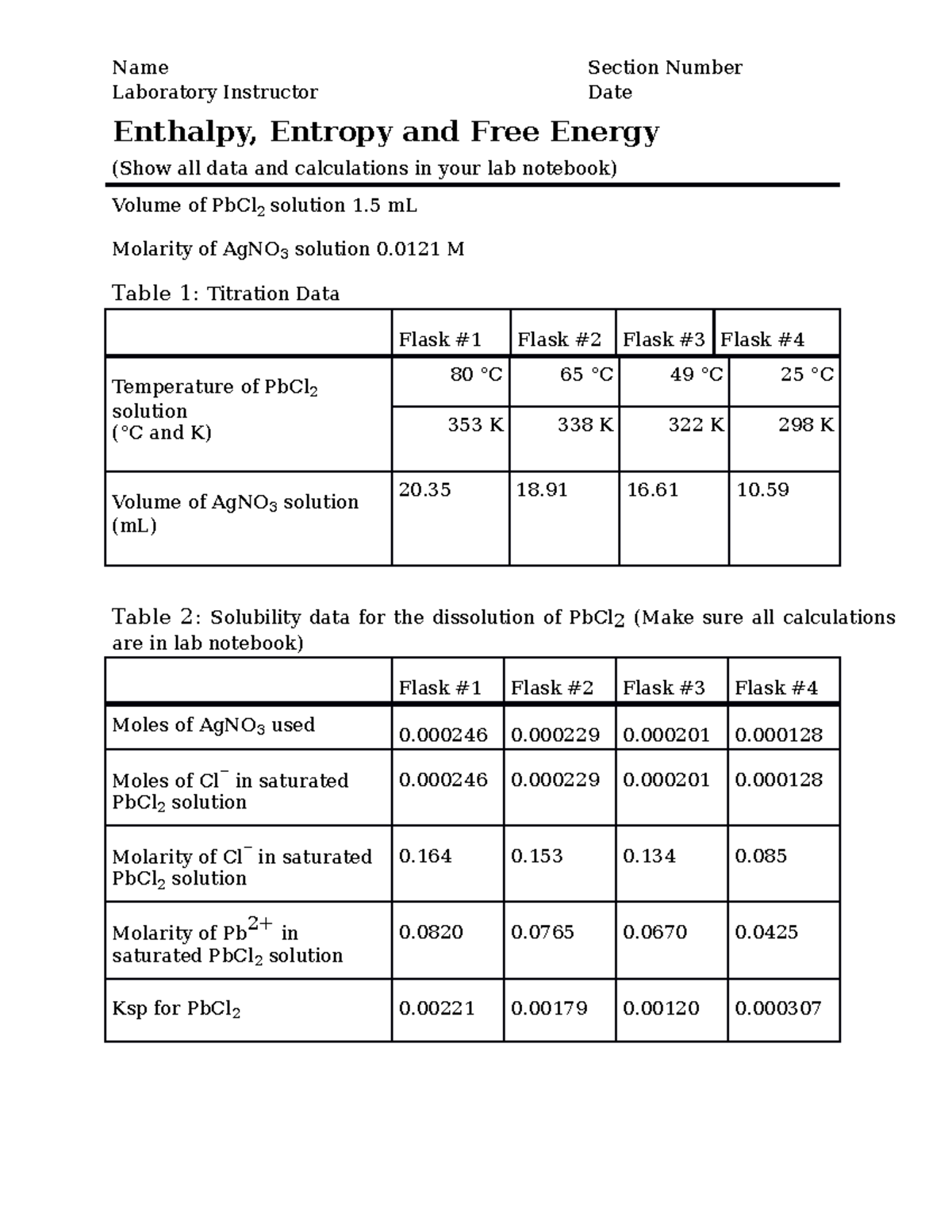
www.e-streetlight.com
Gibbs Free Energy Worksheet Unique Quiz & Worksheet Spontaneous
chessmuseum.org
Is) Quiz-Worksheet-Gibbs-Free-Energy | PDF – Worksheets Library

worksheets.clipart-library.com
Gibbs Free Energy Worksheet – Pro Worksheet

www.proworksheet.my.id
Gibbs Free Energy Worksheet – Pro Worksheet

www.proworksheet.my.id
Gibbs Free Energy Entropy Enthalpy Chart – Ponasa
ponasa.condesan-ecoandes.org
Gibbs Free Energy Worksheet With Answers – Printable Word Searches

davida.davivienda.com
Types of energy worksheet – pro worksheet. Is) quiz-worksheet-gibbs-free-energy. gibbs free energy worksheet – pro worksheet