Understanding the composition of chemical compounds is fundamental to chemistry. A crucial step in this understanding is mastering the ability to count atoms within chemical formulas. This is where “How To Count Atoms” worksheets come into play! These worksheets provide invaluable practice in interpreting chemical formulas and determining the number of atoms of each element present. They are designed to help students grasp the concept of subscripts, coefficients, and polyatomic ions, ultimately building a solid foundation for more advanced chemical calculations and reactions. Let’s delve into how these worksheets work and why they are so important for chemistry learners.
Why Are “How To Count Atoms” Worksheets Important?
Simply put, these worksheets bridge the gap between abstract chemical formulas and the tangible reality of atoms that make up matter. Here’s a breakdown of their significance:
* **Understanding Chemical Formulas:** Chemical formulas are like blueprints for molecules. They contain crucial information about the elements present and their respective quantities. Worksheets train students to decipher these blueprints, recognizing elements by their symbols (e.g., H for Hydrogen, O for Oxygen) and understanding the significance of subscripts.
* **Grasping Subscripts and Coefficients:** Subscripts indicate the number of atoms of a particular element *within* a molecule. For instance, in H2O, the subscript ‘2’ indicates that there are two hydrogen atoms. Coefficients, on the other hand, indicate the number of *molecules* of the compound. For example, 2H2O means there are two molecules of water, each containing two hydrogen atoms and one oxygen atom. The worksheets provide practice in correctly applying these concepts.
* **Working with Polyatomic Ions:** Polyatomic ions are groups of atoms that act as a single unit with an overall charge (e.g., SO42-, sulfate). Learning to recognize and count the atoms within polyatomic ions is essential. Worksheets often include compounds containing these ions, requiring students to carefully distribute subscripts outside the parentheses.
* **Building a Foundation for Stoichiometry:** Counting atoms is a prerequisite for understanding stoichiometry, the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. Stoichiometry relies heavily on the correct interpretation of chemical formulas and the ability to calculate the number of moles of each element. Without a strong foundation in counting atoms, stoichiometric calculations become incredibly difficult.
* **Developing Problem-Solving Skills:** Successfully completing these worksheets requires logical thinking and attention to detail. Students learn to break down complex formulas into smaller, manageable parts, developing critical problem-solving skills that are applicable to a wide range of scientific disciplines.
How To Use a “How To Count Atoms” Worksheet Effectively
To maximize the benefits of these worksheets, follow these tips:
1. **Start with Simple Formulas:** Begin with basic formulas like NaCl (sodium chloride) or CO2 (carbon dioxide). This builds confidence and reinforces the fundamental principles.
2. **Focus on Understanding, Not Memorization:** Instead of rote memorization, focus on understanding the meaning of subscripts and coefficients. Ask yourself, “What does this subscript tell me about the number of atoms?”
3. **Practice, Practice, Practice:** The more you practice, the better you’ll become at recognizing patterns and quickly counting atoms.
4. **Work Through Examples:** Carefully examine solved examples to understand the step-by-step process. Pay attention to how the subscripts are applied and how coefficients are distributed.
5. **Don’t Be Afraid to Ask for Help:** If you’re struggling with a particular concept, don’t hesitate to ask your teacher or a classmate for clarification.
6. **Use a Periodic Table:** Keep a periodic table handy to easily identify the elements represented by their symbols.
Example: How To Count Atoms in Ca3(PO4)2
Let’s break down the process of counting atoms in Calcium Phosphate, Ca3(PO4)2:
* **Calcium (Ca):** The subscript ‘3’ indicates that there are 3 atoms of calcium.
* **Phosphorus (P):** The subscript ‘2’ outside the parentheses multiplies the number of phosphorus atoms inside the parentheses. There is ‘1’ phosphorus atom inside the parentheses (remember, if there is no subscript, it is assumed to be ‘1’). Therefore, there are 1 x 2 = 2 atoms of phosphorus.
* **Oxygen (O):** Similarly, the ‘2’ outside the parentheses multiplies the number of oxygen atoms inside the parentheses. There are ‘4’ oxygen atoms inside the parentheses, so there are 4 x 2 = 8 atoms of oxygen.
Therefore, in one molecule of Ca3(PO4)2, there are 3 calcium atoms, 2 phosphorus atoms, and 8 oxygen atoms.
By consistently practicing with “How To Count Atoms” worksheets and understanding the principles behind them, you’ll gain a crucial skill that will serve you well throughout your chemistry studies.
Answer Example for a “How To Count Atoms” Worksheet
Here’s an example of how a “How To Count Atoms” worksheet might be structured and the corresponding answers in HTML list format:
Instructions: Count the number of atoms of each element in the following chemical formulas.
Chemical Formula
- H2O
- NaCl
- MgCl2
- 2H2SO4
- Al2(SO4)3
Answer
- Hydrogen: 2, Oxygen: 1
- Sodium: 1, Chlorine: 1
- Magnesium: 1, Chlorine: 2
- Hydrogen: 4, Sulfur: 2, Oxygen: 8
- Aluminum: 2, Sulfur: 3, Oxygen: 12
Answer List in HTML:
Answers:
- H2O:
- Hydrogen (H): 2
- Oxygen (O): 1
- NaCl:
- Sodium (Na): 1
- Chlorine (Cl): 1
- MgCl2:
- Magnesium (Mg): 1
- Chlorine (Cl): 2
- 2H2SO4:
- Hydrogen (H): 4
- Sulfur (S): 2
- Oxygen (O): 8
- Al2(SO4)3:
- Aluminum (Al): 2
- Sulfur (S): 3
- Oxygen (O): 12
If you are looking for HW 3 Counting atoms in compounds w.s | Science, Chemistry, Atoms you’ve came to the right web. We have 20 Images about HW 3 Counting atoms in compounds w.s | Science, Chemistry, Atoms like How to Count atoms Worksheet Awesome Counting atoms Worksheet Answers, How To Count Atoms Worksheet and also Kami Export – Miracle Ikwuakolam – Chemistry 1 Classification of. Read more:
HW 3 Counting Atoms In Compounds W.s | Science, Chemistry, Atoms

worksheets.clipart-library.com
Counting Atoms 1 Worksheet Answers – CountingWorksheets.com

www.countingworksheets.com
Counting Atoms Worksheet
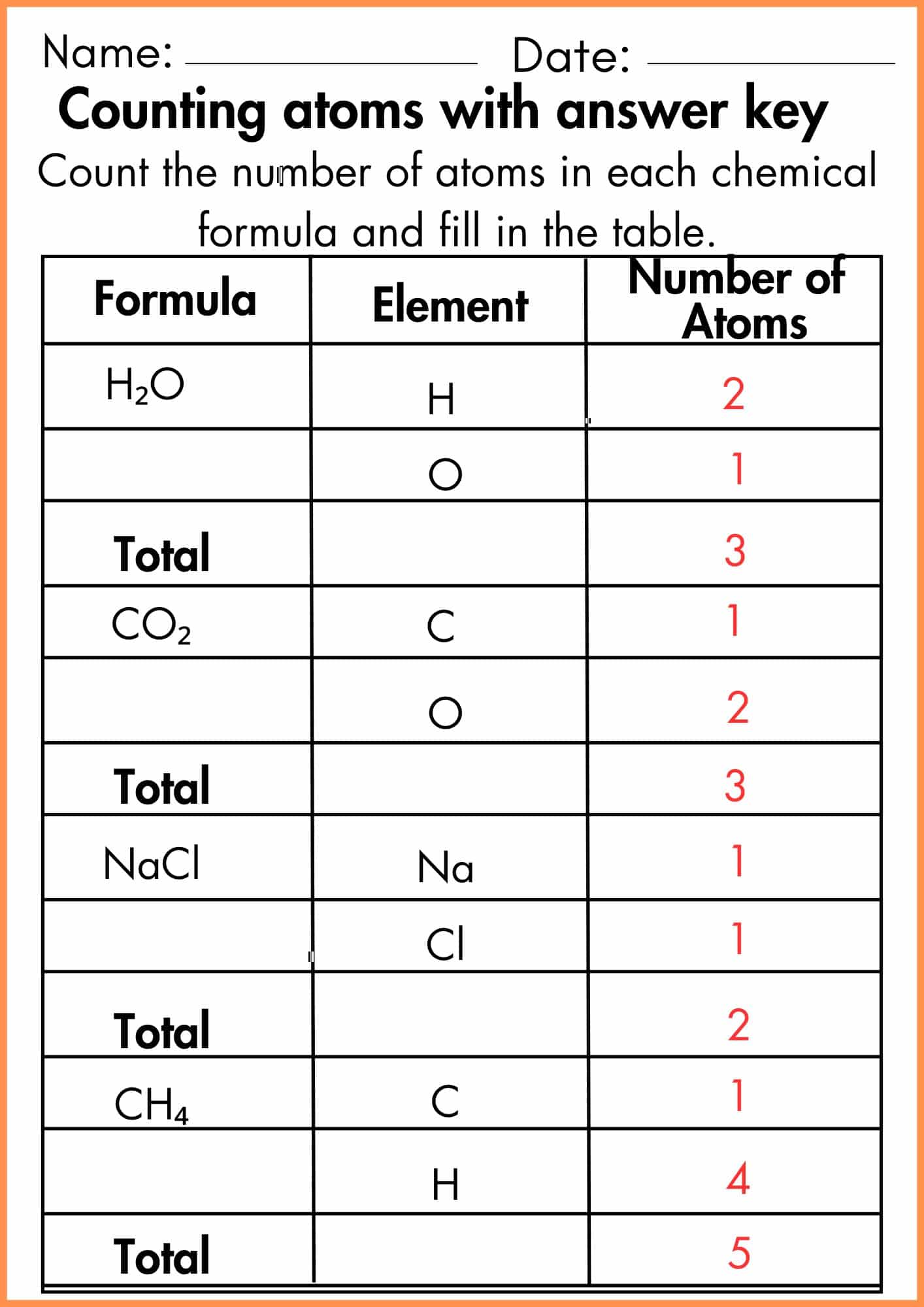
eduinput.com
How To Count Atoms Worksheet Inspirational Showme Counting Atoms In
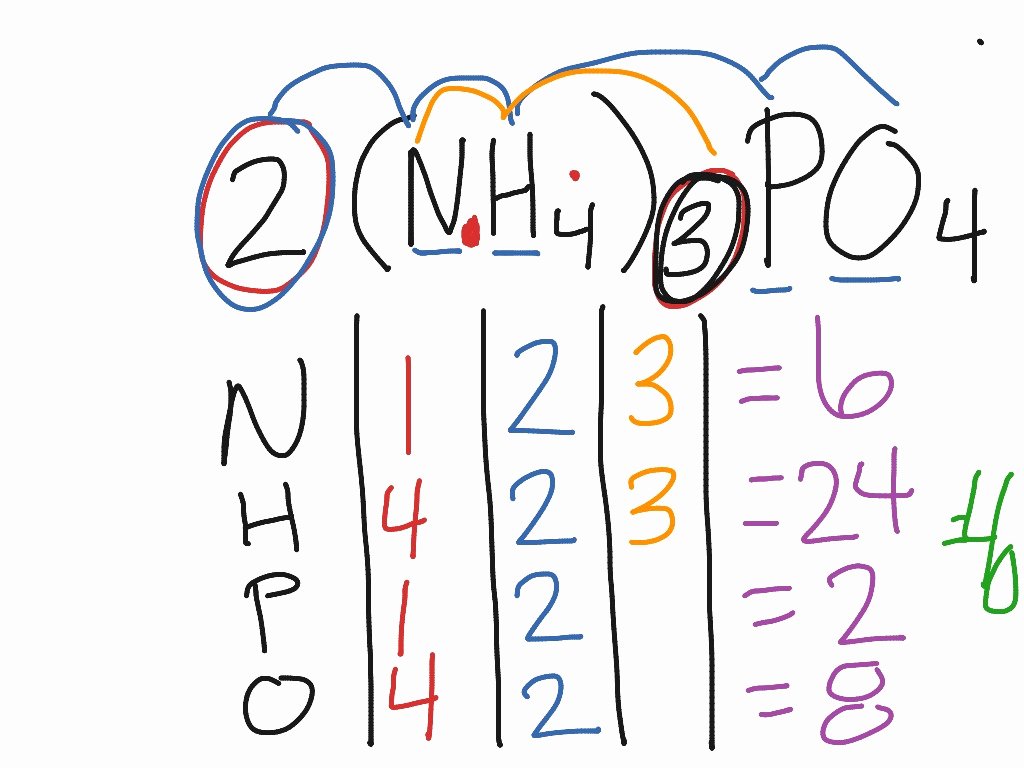
chessmuseum.org
Atoms And Elements Worksheet – E-streetlight.com
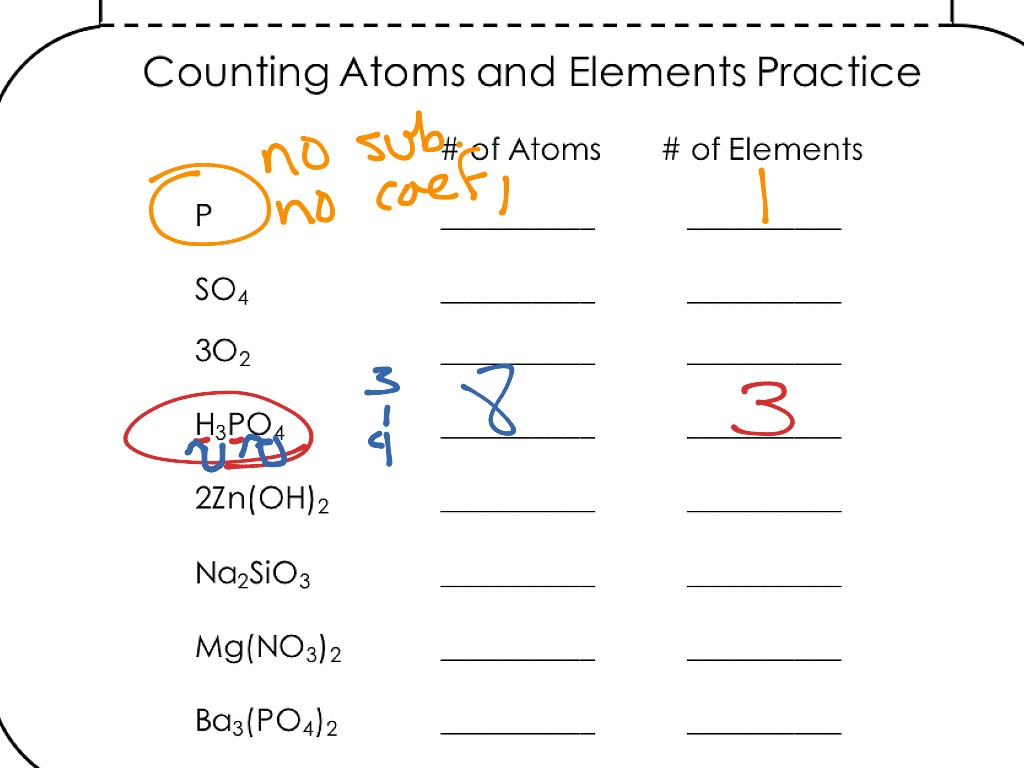
www.e-streetlight.com
Counting Atoms Worksheet Answer Key – E-streetlight.com
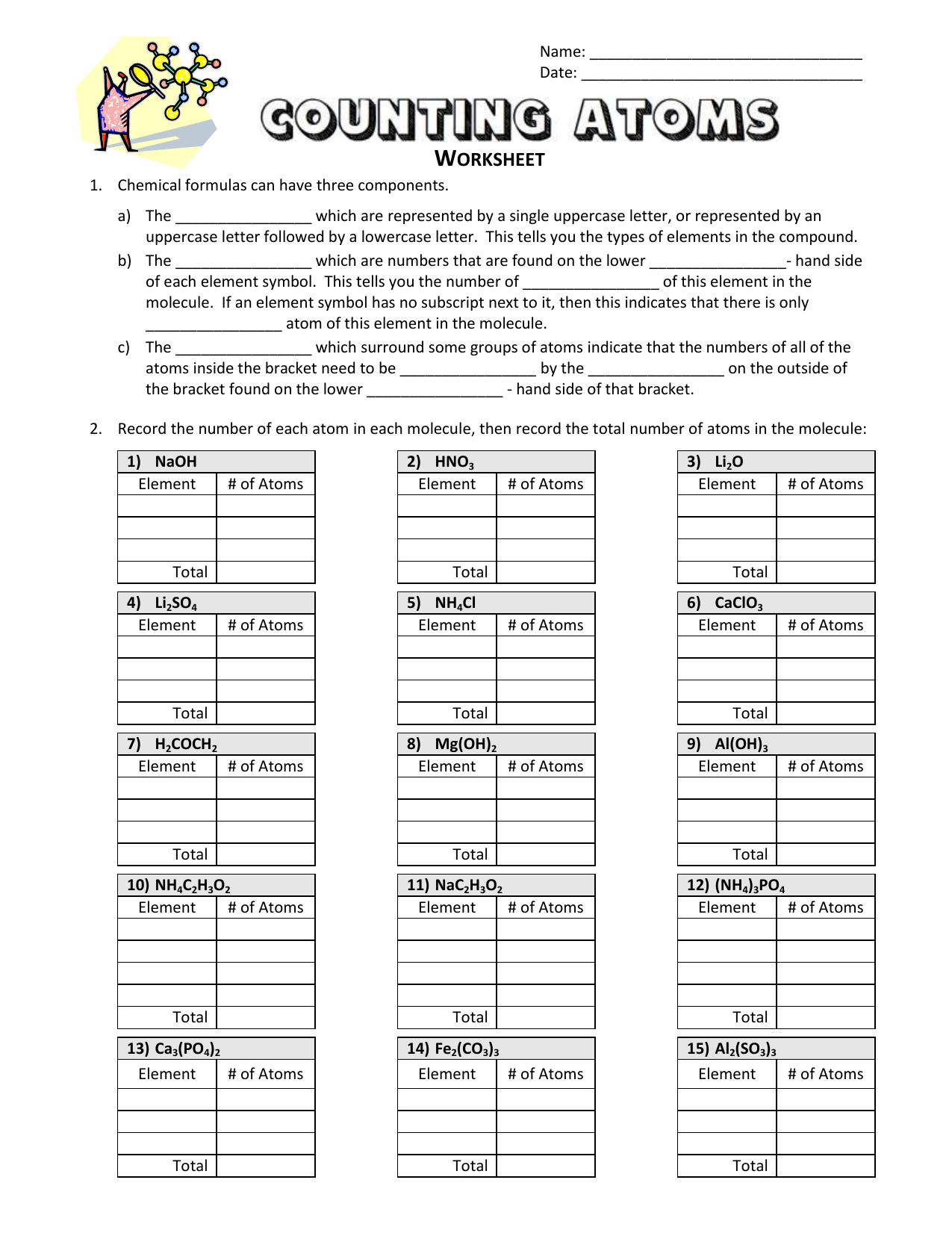
www.e-streetlight.com
Kami Export – Miracle Ikwuakolam – Chemistry 1 Classification Of

worksheets.clipart-library.com
Free Printable Counting Atoms Worksheet With Answers [Practice, Color

www.typecalendar.com
Elements Compounds With Two Or More Atoms Storyboard
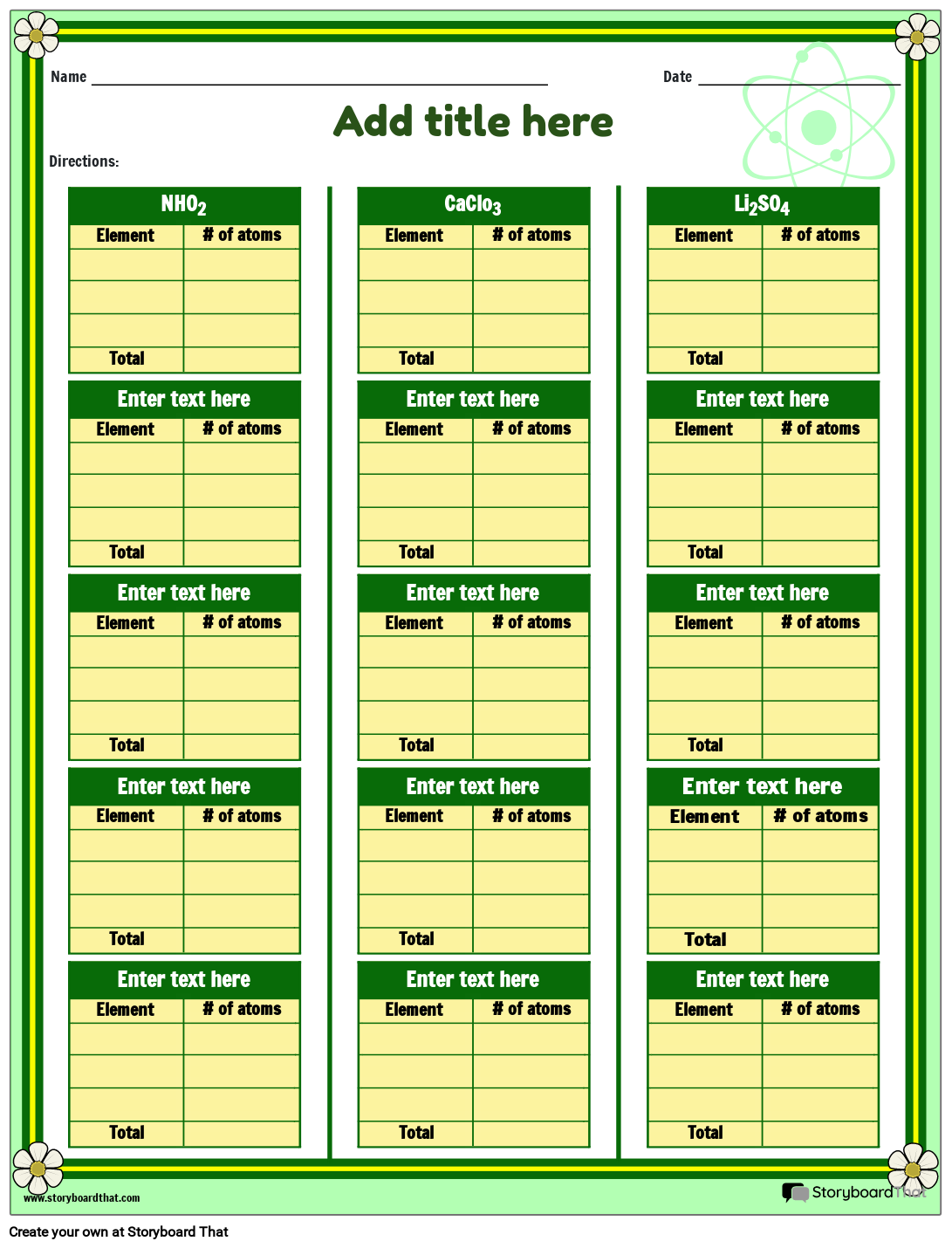
www.storyboardthat.com
Free Printable Counting Atoms Worksheet With Answers [Practice, Color

www.typecalendar.com
10 Best Counting Atoms Worksheets For Learning Atomic Structure

worksheets.clipart-library.com
How To Count Atoms Worksheet – E Street Light

www.e-streetlight.com
How To Count Atoms Worksheet – E Street Light
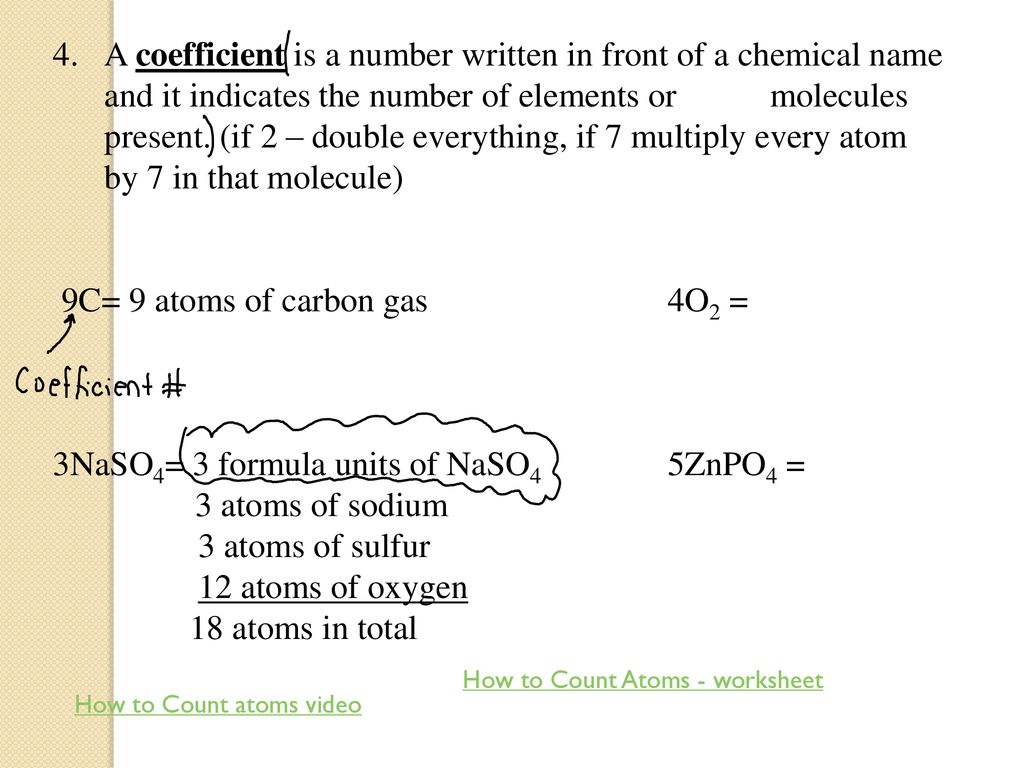
www.e-streetlight.com
How To Count Atoms Worksheet – E Street Light

www.e-streetlight.com
How To Count Atoms Worksheet New 17 Best Of Counting Atoms Worksheet
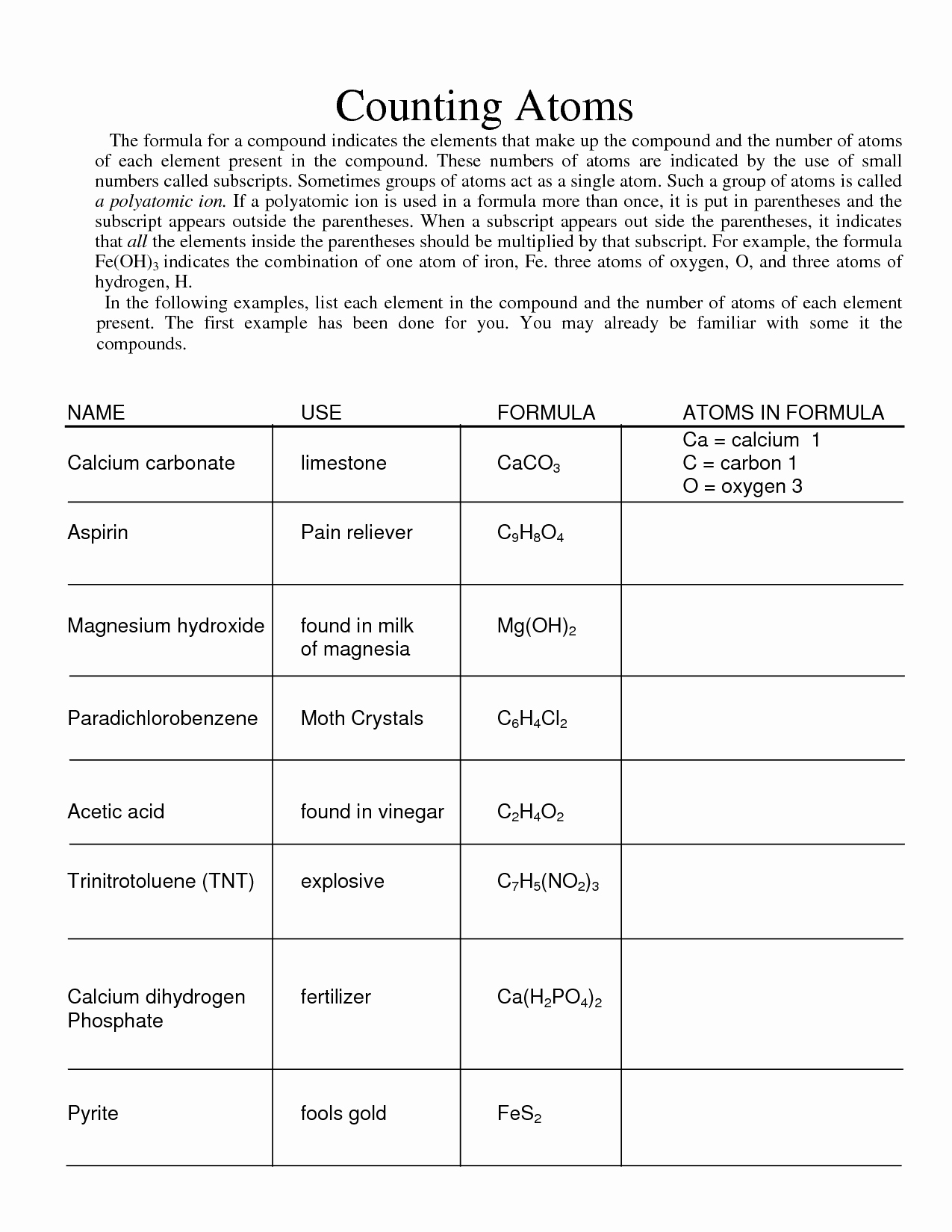
chessmuseum.org
Counting Atoms Practice Worksheet – Printable Word Searches

davida.davivienda.com
10 Best Counting Atoms Worksheets For Learning Atomic Structure – The

teachsimple.com
How To Count Atoms Worksheet Awesome Counting Atoms Worksheet Answers
chessmuseum.org
How To Count Atoms Worksheet

www.proworksheet.my.id
How To Count Atoms Worksheet – Pro Worksheet

www.proworksheet.my.id
how to count atoms worksheet – pro worksheet. 10 best counting atoms worksheets for learning atomic structure. How to count atoms worksheet new 17 best of counting atoms worksheet