Okay, let’s tackle the beast that is molarity practice! Molarity, a fundamental concept in chemistry, expresses the concentration of a solution. It tells us the number of moles of solute (the substance being dissolved) present in one liter of solution. Understanding molarity is absolutely crucial for a wide range of applications, from preparing solutions in the lab to understanding chemical reactions in biological systems. Many students find molarity calculations tricky at first, often getting tripped up by unit conversions or confusing the definitions of solute, solvent, and solution. That’s why practicing with worksheets is so essential! This post aims to provide clarity on some common molarity practice problems and, of course, present some answers to help you check your work. Remember, understanding the *process* of solving these problems is far more important than just memorizing the answers. So, use these answers as a guide to understanding, not a shortcut to completion.
Understanding Molarity: A Quick Recap
Before diving into the answers, let’s quickly review the key concepts. The formula for molarity is:
Molarity (M) = Moles of Solute / Liters of Solution
Remember these important points:
- Solute: The substance being dissolved (e.g., salt in saltwater).
- Solvent: The substance doing the dissolving (e.g., water in saltwater).
- Solution: The homogeneous mixture formed by the solute and solvent (e.g., saltwater).
- Moles: A unit of measurement representing 6.022 x 1023 particles (Avogadro’s number). You often need to convert grams to moles using the molar mass of the solute.
- Liters: The unit of volume for the *solution*, not just the solvent. Make sure you convert milliliters (mL) to liters (L) by dividing by 1000.
Common problem types involve:
- Calculating molarity given the mass of solute and volume of solution.
- Calculating the mass of solute needed to prepare a solution of a specific molarity and volume.
- Calculating the volume of solution needed to contain a specific number of moles of solute.
- Dilution problems using the formula: M1V1 = M2V2
Molarity Practice Worksheet Answers
Here are the answers to some typical molarity practice problems. Please note that your specific worksheet may have slightly different problems, but the principles remain the same. Make sure you show your work to earn full credit on any assignments! Also, significant figures matter! Pay attention to the numbers given in the problems to determine the correct number of significant figures in your answers.
- Question 1: What is the molarity of a solution containing 3 moles of NaCl in 2 liters of solution?
- Answer: 1.5 M
- Question 2: What is the molarity of a solution containing 58.44 grams of NaCl in 1 liter of solution? (Molar mass of NaCl = 58.44 g/mol)
- Answer: 1 M
- Question 3: How many grams of KCl are needed to prepare 500 mL of a 0.2 M solution? (Molar mass of KCl = 74.55 g/mol)
- Answer: 7.46 g
- Question 4: What volume, in mL, of a 2.0 M solution of glucose is needed to obtain 0.1 moles of glucose?
- Answer: 50 mL
- Question 5: If you dilute 25 mL of a 3.0 M solution of HCl to 500 mL, what is the molarity of the diluted solution?
- Answer: 0.15 M
- Question 6: A solution contains 20.0 g of NaOH in 250.0 mL of solution. Calculate the molarity. (Molar mass of NaOH = 40.00 g/mol)
- Answer: 2.00 M
- Question 7: How many moles of H2SO4 are present in 1.5 L of a 0.5 M solution?
- Answer: 0.75 moles
- Question 8: Calculate the mass of solute required to prepare 250 mL of a 0.1 M solution of copper(II) sulfate, CuSO4. (Molar mass of CuSO4 = 159.61 g/mol)
- Answer: 3.99 g
Remember to always double-check your units and make sure you’re using the correct molar mass for the solute. If you’re still struggling, don’t hesitate to ask your teacher or a classmate for help. Chemistry can be challenging, but with practice and persistence, you can master molarity!
If you are looking for Molarity Worksheet 1 Answer Key – properinspire you’ve came to the right place. We have 20 Pics about Molarity Worksheet 1 Answer Key – properinspire like Molarity Practice Worksheet Answer – Pro Worksheet, SOLUTION: Chemistry homework help calculating molarity worksheet and also Molarity Worksheet Answer Key – E-streetlight.com. Here you go:
Molarity Worksheet 1 Answer Key – Properinspire

properinspire.blogspot.com
Answer Key (video) For Worksheet 5.1 (molarity) | Science

worksheets.clipart-library.com
L4 Molarity Practice Worksheet – Molarity Practice Worksheet Find The

www.studocu.com
Molarity Worksheet Answer Key – E-streetlight.com

www.e-streetlight.com
Molarity Molality – Worksheets Library

worksheets.clipart-library.com
Empirical And Molecular Formula Worksheet ANSWER KEY | Study Notes

www.docsity.com
Molarity Worksheet 1 Answer Key – Properinspire

properinspire.blogspot.com
Molarity Practice Worksheet Answer – Pro Worksheet

www.proworksheet.my.id
Molarity Worksheet #1 Answer Key

preseni6qlibguide.z13.web.core.windows.net
Molarity POGIL Key | PDF | Molar Concentration | Solution – Worksheets

worksheets.clipart-library.com
Density Practice Worksheet 1 Pdf Density Practice Wor – Vrogue.co

www.vrogue.co
Free Printable Molarity Worksheets – Worksheets Library

worksheets.clipart-library.com
Molarity Worksheet Answer Key – E-streetlight.com

www.e-streetlight.com
Molarity Molality Worksheet.pdf – Name Per Concentrations

worksheets.clipart-library.com
Molarity Practice Worksheet 1-3 | Science, Chemistry, Solutions
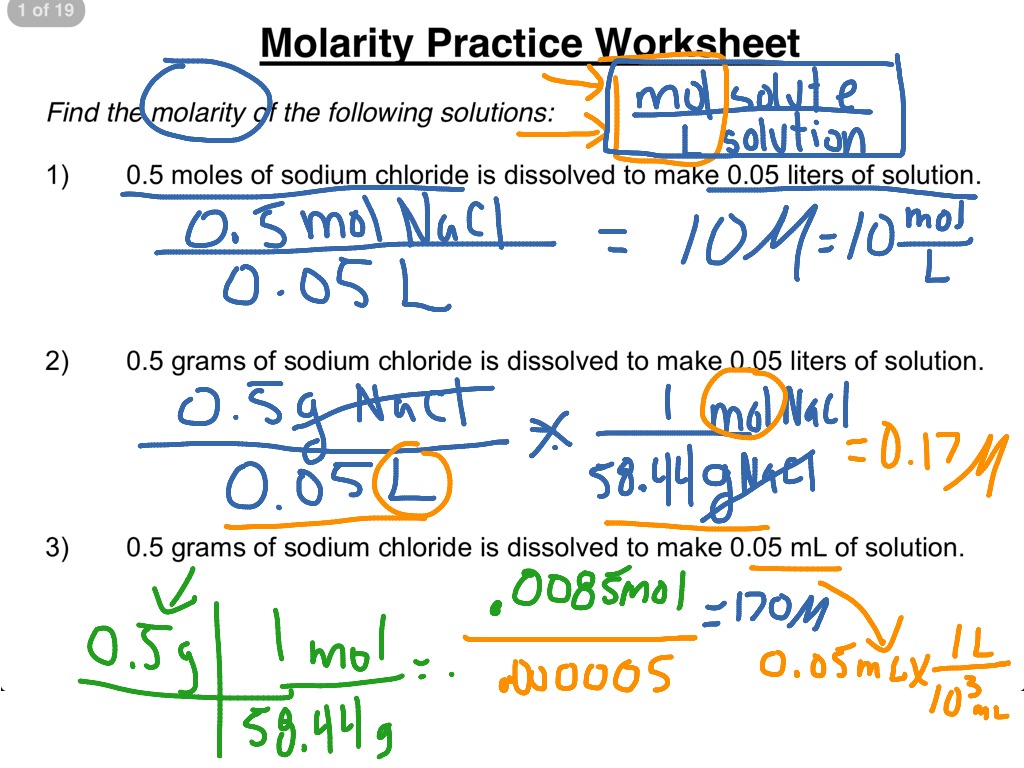
worksheets.clipart-library.com
Molarity Practice Worksheet Answer – Pro Worksheet

www.proworksheet.my.id
Molarity Practice Worksheet – Molarity Practice Worksheet Names: Date
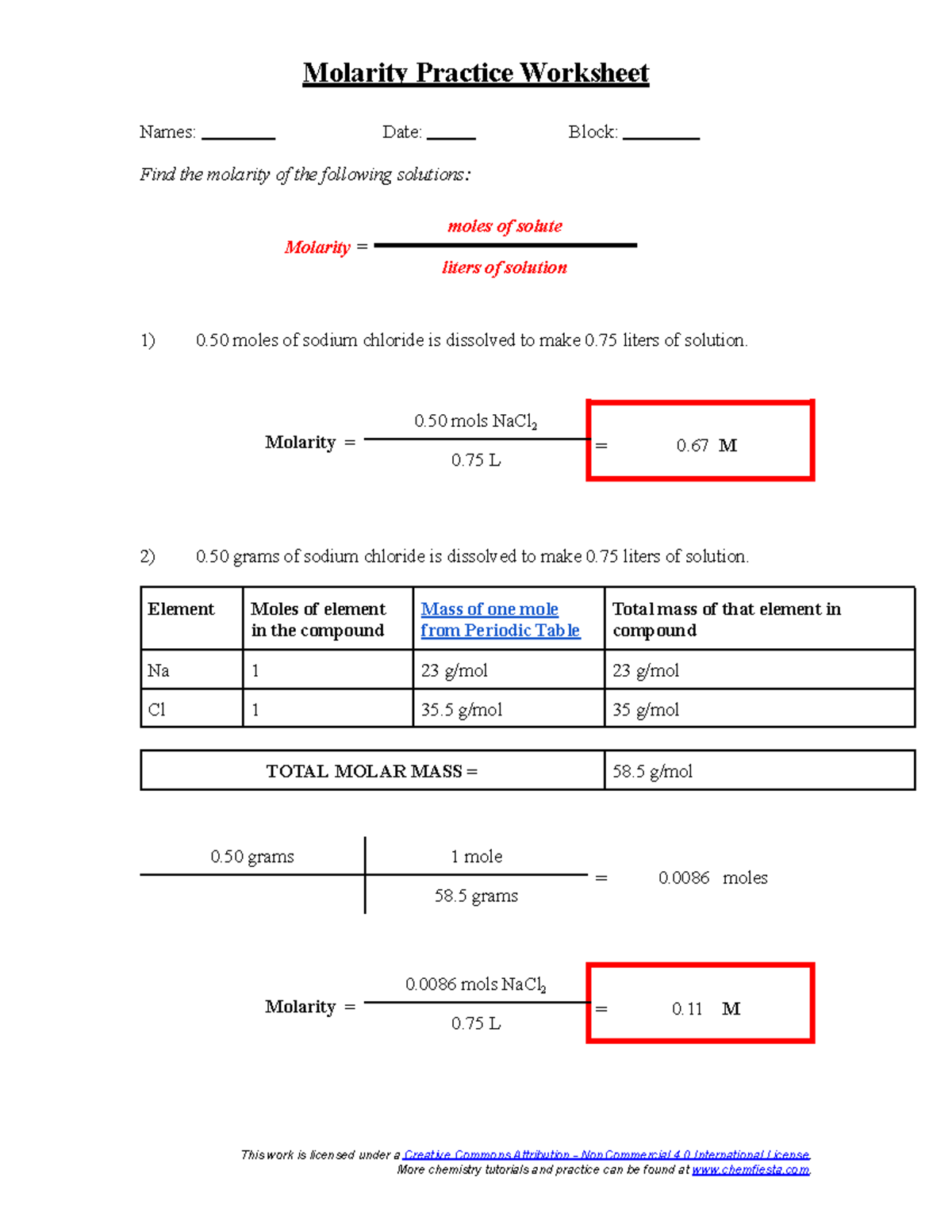
www.studocu.com
Molarity Molality Concentration Worksheets KEY | PDF – Worksheets Library

worksheets.clipart-library.com
Molarity Worksheet Answer Key – Pro Worksheet
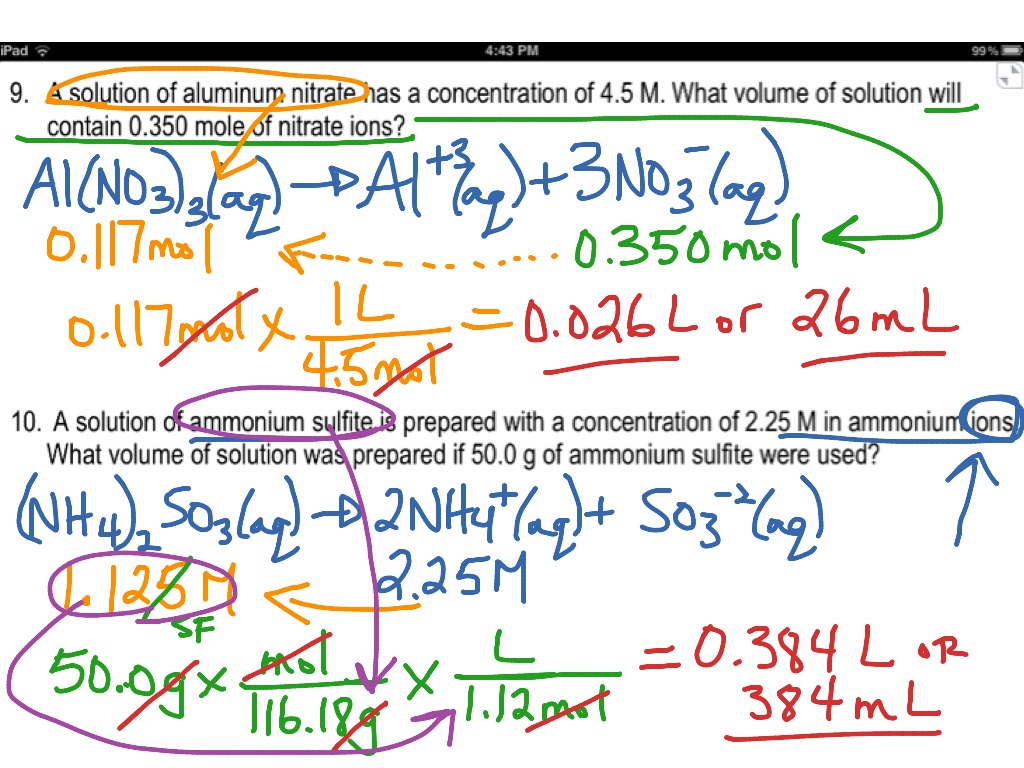
www.proworksheet.my.id
SOLUTION: Chemistry Homework Help Calculating Molarity Worksheet

worksheets.clipart-library.com
Molarity practice worksheet. Density practice worksheet 1 pdf density practice wor. Molarity molality worksheet.pdf