Alright, budding chemists! Are you tackling quantum numbers and feeling a bit… uncertain? Fear not! Understanding quantum numbers is crucial for grasping the behavior of electrons within atoms, and that knowledge unlocks a deeper understanding of chemical bonding and reactivity. Mastering these numbers can seem like deciphering a secret code, but with practice and a clear understanding of the underlying principles, you’ll be fluent in electron-speak in no time. This post focuses on using practice worksheets to solidify your understanding of quantum numbers and then provides answers to a typical practice worksheet. Work through the problems yourself first, and then check your work against the answers provided below. Remember, the goal isn’t just to get the right answer but to understand *why* the answer is correct.
Think of quantum numbers as a set of coordinates that define the “address” of an electron within an atom. Each electron has a unique set of four quantum numbers that describe its energy, shape, spatial orientation, and spin. These four numbers are:
- Principal Quantum Number (n): This number describes the energy level of the electron. It’s a positive integer (n = 1, 2, 3, …), with higher numbers indicating higher energy levels and greater distance from the nucleus. Think of it like the floor number in a building; higher floors require more energy to reach.
- Azimuthal or Angular Momentum Quantum Number (l): This number describes the shape of the electron’s orbital. Its values range from 0 to n-1. l = 0 corresponds to an s orbital (spherical), l = 1 corresponds to a p orbital (dumbbell-shaped), l = 2 corresponds to a d orbital (more complex shapes), and l = 3 corresponds to an f orbital (even more complex shapes). Imagine these shapes as different types of rooms on each floor.
- Magnetic Quantum Number (ml): This number describes the orientation of the orbital in space. Its values range from -l to +l, including 0. For example, if l = 1 (a p orbital), then ml can be -1, 0, or +1, representing the three different p orbitals oriented along the x, y, and z axes. These are like different orientations of the same type of room.
- Spin Quantum Number (ms): This number describes the intrinsic angular momentum of the electron, which is quantized and referred to as “spin.” Electrons behave as if they are spinning, creating a magnetic dipole moment. The spin quantum number can only have two values: +1/2 (spin up) or -1/2 (spin down). Think of this as the direction the electron is spinning.
Understanding the allowed values for each quantum number is the key to predicting and explaining electron configurations and, ultimately, the chemical properties of elements. So, grab your practice worksheet, a pencil, and let’s dive in!
Quantum Numbers Practice Worksheet Answers
Below are the answers to a typical Quantum Numbers Practice Worksheet. Remember to work through the problems on your own first to truly test your understanding!
Example Problems and Solutions
-
Question: What are the possible values of *l* if *n* = 3?
Answer:
- *l* can range from 0 to *n*-1. Therefore, if *n* = 3, *l* can be 0, 1, or 2.
-
Question: If *l* = 2, what are the possible values of *ml*?
Answer:
- *ml* can range from -*l* to +*l*. Therefore, if *l* = 2, *ml* can be -2, -1, 0, 1, or 2.
-
Question: What type of orbital is described by the quantum numbers *n* = 4, *l* = 1?
Answer:
- *n* = 4 indicates the 4th energy level. *l* = 1 corresponds to a p orbital. Therefore, this describes a 4p orbital.
-
Question: Is the following set of quantum numbers possible: *n* = 2, *l* = 2, *ml* = 0, *ms* = +1/2?
Answer:
- No, this set is not possible. If *n* = 2, the possible values of *l* are 0 and 1. *l* = 2 is not allowed.
-
Question: Give a possible set of quantum numbers for the last electron added to the nitrogen atom when building its electron configuration from the ground state. (Nitrogen has 7 electrons).
Answer:
- Nitrogen’s electron configuration is 1s22s22p3. The last electron added is one of the 2p electrons.
A possible set of quantum numbers is:- *n* = 2 (2nd energy level)
- *l* = 1 (p orbital)
- *ml* = -1, 0, or +1 (any of the p orbitals is acceptable since we are only asked for “a” possible set)
- *ms* = +1/2 or -1/2 (either spin is acceptable)
So, a complete and acceptable answer would be: *n*=2, *l*=1, *ml*=0, *ms*= +1/2. Many other combinations are also correct.
- Nitrogen’s electron configuration is 1s22s22p3. The last electron added is one of the 2p electrons.
This example practice worksheet provides a good starting point. You can find many more online or in your textbook. The key is to keep practicing and to understand the relationships between the quantum numbers. Good luck, and happy quantum-ing!
If you are searching about Quantum Numbers Worksheet Answers – Pro Worksheet you’ve came to the right web. We have 20 Pics about Quantum Numbers Worksheet Answers – Pro Worksheet like Quantum Paper – Worksheet, Solutions – Chemistry LibreTexts – Worksheets Library and also Quantum Numbers Practice Worksheet – Ame.my.id. Here it is:
Quantum Numbers Worksheet Answers – Pro Worksheet

www.proworksheet.my.id
Quantum Numbers Practice Worksheet – Ame.my.id
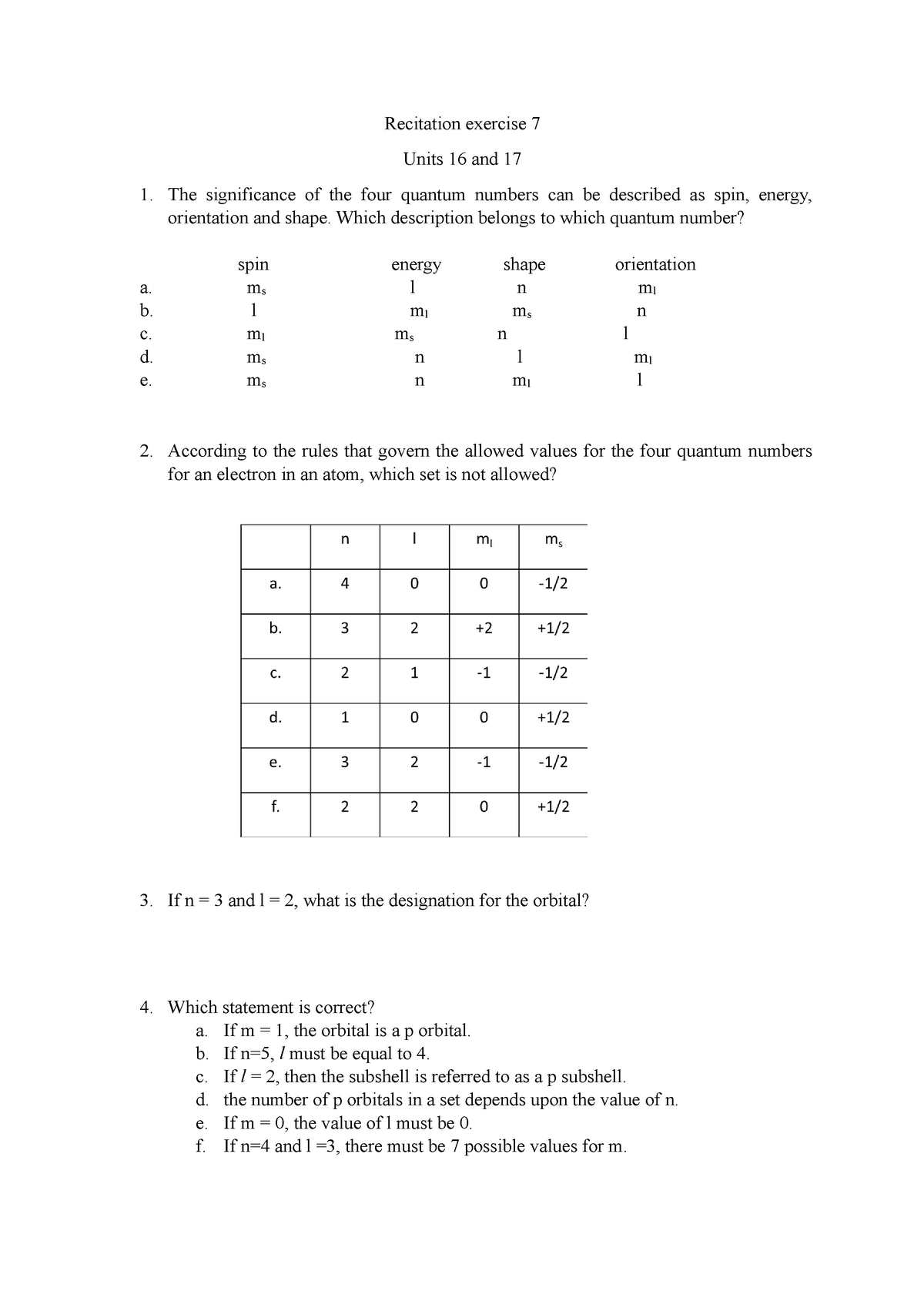
ame.my.id
Quantum Numbers Practice Worksheet – Owhentheyanks.com

www.owhentheyanks.com
Quantum Physics – Worksheets Library

worksheets.clipart-library.com
SOLUTION: Electron Configuration And Quantum Numbers Worksheet – Studypool

www.studypool.com
Quantum Numbers Practice Worksheet – Owhentheyanks.com
www.owhentheyanks.com
Orbitals And Quantum Numbers Practice Questions | Study Notes Quantum

www.docsity.com
Quantum Number Worksheet With Answers At Gethughblog Blog

gethughblog.blob.core.windows.net
Quantum Mechanics Crossword Puzzle Worksheet Activity | Teaching
worksheets.clipart-library.com
Quantum Numbers Worksheet Answers – E-streetlight.com

www.e-streetlight.com
Solutions – Chemistry LibreTexts – Worksheets Library
worksheets.clipart-library.com
How Do You Teach The Quantum Numbers Of Carbon? — CoScine Creative

worksheets.clipart-library.com
Quantum Numbers Practice Worksheet

www.pinterest.com
SOLUTION: Quantum Numbers Worksheet – Studypool

www.studypool.com
Quantum Paper – Worksheet

quantumpaper.in
Quantum Numbers Worksheet – Name

www.studocu.com
SOLUTION: Quantum Numbers Worksheet (With Answers) – Studypool

www.studypool.com
Quantum Numbers Practice Worksheet – Owhentheyanks.com
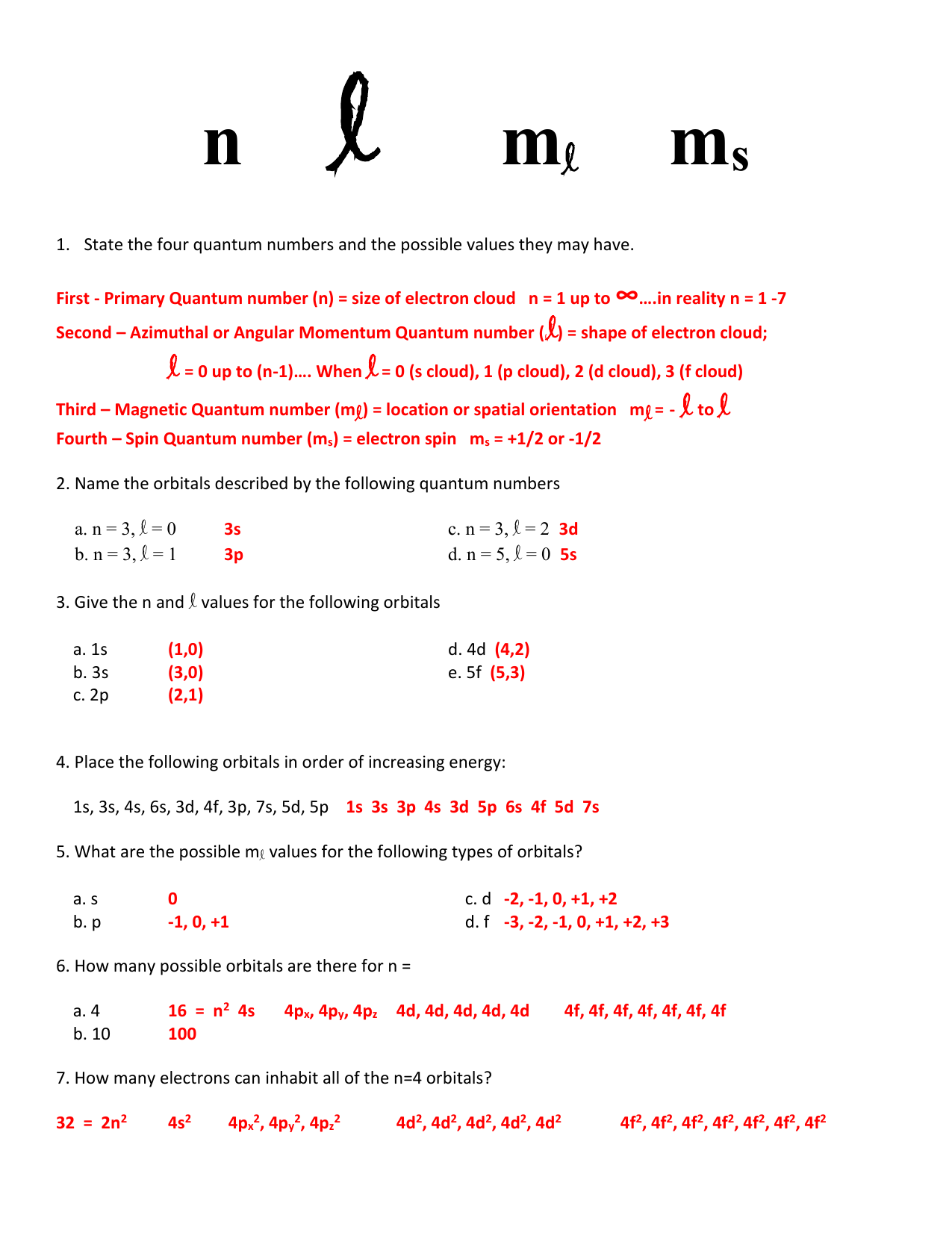
www.owhentheyanks.com
Quantum Numbers Worksheet – Structure And Properties Name: Azhar

www.studocu.com
Quantum Numbers Practice Worksheet – Printable PDF Template

martinlindelof.com
quantum numbers practice worksheet. Quantum numbers practice worksheet – printable pdf template. quantum numbers practice worksheet – owhentheyanks.com